Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
kLa Determination in Shake Flasks
You will learn what kLa means, what methods there are to measure it and what possible set-ups PreSens offers you. You can also use our kLa Calculator tool, if you are ready for measurements.
kLa Determination in Shake Flasks
The kLa is a measure of the aeration capacity of a bioreactor system for a given set of operating conditions. It is given in the unit [h-1]. A larger kLa value indicates higher aeration capacity of the bioreactor system. The kLa in Erlenmeyer flasks is depending on different factors, the most important being the applied volume and the shaking speed. Further parameters like temperature and media composition can also affect the kLa, but only to a negligible extent.
You can determine the kLa value for your shake flasks in your specific operating conditions either using the gassing-out method or if gassing-out is not possible and for buffers the sulfite method.
Gassing-Out Method
This method is based on monitoring the increase of dissolved oxygen concentration of a solution during aeration and agitation. The oxygen transfer rate (OTR) will decrease during the aeration period as the dissolved oxygen concentration in the liquid ([O2]L) will reach saturation concentration ([O2]*).
- Prepare a flask of the same type and size, and fill it with the same volume of culture broth or distilled water you are going to use for your culture monitoring later on. Place it inside your shaking incubator, perform the respective O2 sensor calibration and start oxygen monitoring.
- Decrease the oxygen content inside the liquid by gassing it with nitrogen.
- When the oxygen inside the liquid is removed, stop gassing it with nitrogen and replace the nitrogen in the headspace with air. Start aeration by agitating the liquid. The agitation speed should match the rpm you are going to use in the following culture monitoring experiments. Monitor the increase in dissolved oxygen concentration.
- The increase in dissolved oxygen concentration is given by:
d [O2]L / d t = kLa ([O2]* - [O2]L)
Taking logarithm after integration of the equation gives:
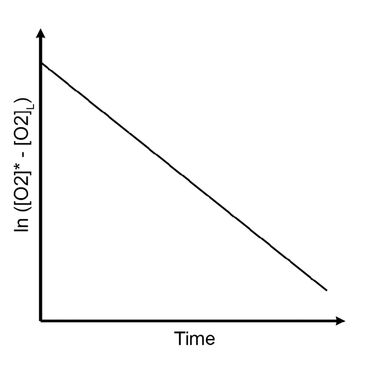
ln ([O2]* - [O2]L) = - kLa * t
Plot the ln ([O2]* - [O2]L) against the aeration time, which will give you a straight line (see Fig. left). The slope of the plot equals – kLa.
Sulfite Method
This method is used when determining kLa in buffer. It is not recommended for kLa determination in organic media compositions (for kLa determination in media use the gassing-out method described above).
- Fill your flask with buffer until 90 % of your experimental filling volume is reached.
- Prepare add-on solution by dissolving 10 g of sodium sulfite (Na2SO3) and 500 µL cobalt nitrate (CO(NO3)2) standard solution (p(CO) = 1000 mg/L; in nitric acid 0,5 mol/L) in 100 mL water.
- Place the flask inside your shaking incubator and wait until the buffer has equilibrate. Perform O2 sensor calibration at 100% air saturation and start oxygen monitoring.
- Add 10% the amount of your filling volume of add-on solution (see #2) to buffer. The sodium sulfite will consume the oxygen inside the buffer, and you can observe a decrease in oxygen concentration.
- Then start agitating the liquid and measure the oxygen concentration using fast measurement rate. The agitation speed should match the rpm you are going to use in the following culture monitoring experiment. After all sodium sulfite is oxidized, dissolved oxygen will increase. Monitor the increase in dissolved oxygen concentration until your starting concentration is reached.
- The increase in dissolved oxygen concentration is given by:
d [O2]L / d t = kLa ([O2]* - [O2]L)
Taking logarithm after integration of the equation gives:
ln ([O2]* - [O2]L) = - kLa * t
Plot the ln ([O2]* - [O2]L) against the aeration time, which will give you a straight line (see Fig. left). The slope of the plot equals – kLa.
Multi-channel Set-up to Determine kLa in Erlenmeyer Flasks
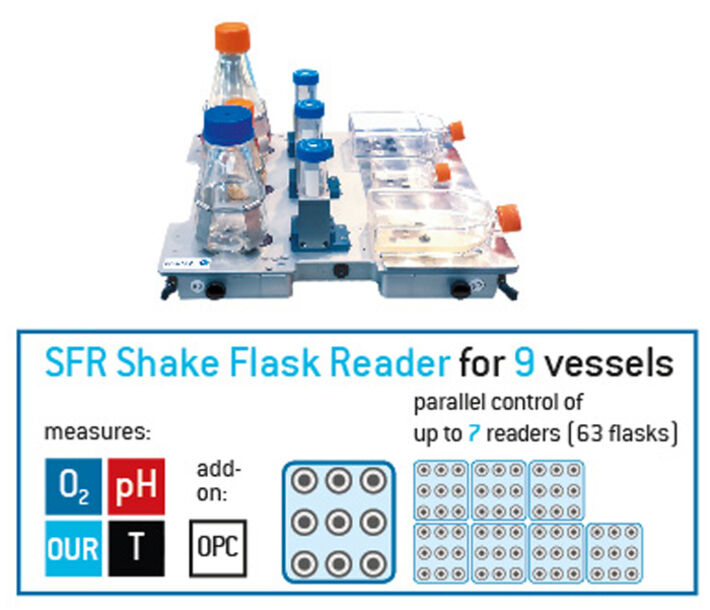
Using chemical optical sensors, it is easy to monitor oxygen in shake flasks. As described above the kLa is calculated from the oxygen change in the Erlenmeyer flasks over time. PreSens offers two different system (Figures below): The SFR vario, which monitors and simultaneously measures O2, pH, biomass and optionally CO2, and the SFR Shake Flask Reader which measures pH and oxygen in up to 9 flasks. The control software of SFR vario allows operating up to 4 devices simultaneously, so up to 4 flasks can be read out in parallel. With the SFR Shake Flask Reader up to 7 systems can be linked together, resulting in a total of up to 63 flasks that can be monitored at once. Both measurement systems offer automatic OUR calculation and can be used for monitoring in Erlenmeyer flasks, cultivation tubes, or T-flasks.