Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Accurate Insight into Oxygen Content of Shake Cultures
Michael Findeis and Gernot T. John
PreSens GmbH, 93053 Regensburg, Germany
Numerous factors affect growth in cell culture in shake flasks. The free oxygen content plays a decisive role. Insufficient oxygen input from the environment limits growth. With the new SFR measurement system the dissolved oxygen content of the medium can be determined non-invasively and extremely accurately. Continuous oxygen monitoring offers advantages for growing new sensitive cell lines, for media optimisation and for QA-related process control.
Non-contact Oxygen Measurement
Key parameters in aerobic cell culture are the dissolved oxygen content and the pH of the medium, both of which decisively affect the culture quality. The two values change over the course of cell culture due to oxygen consumption and acidification of the medium through products of metabolism. Continuous monitoring of oxygen consumption and pH is advantageous for optimisation of cell culture processes. If certain limits are exceeded the corresponding corrections, e.g. rapid changes of the medium or the partial pressure of oxygen, can be made. Reliable information about the metabolic state of the culture is essential for quality assurance.
Non-contact measurement of oxygen content and pH via non-invasive optical sensors (1) is now also available for shake cultures (Fig. 1), making sustainable improvement of the process possible. The optoelectronic measuring apparatus of the SFR is based on optical chemical sensors. Unlike predecessor systems, the new SFR technique offers combined measurement of DO and pH. The measuring principle of the oxygen sensor is based on selective recording of the simple sensor luminescence. In the pH sensor the luminescence is detected by means of a dual-signal process with an additional integrated reference sensor. Sensor readout is performed using modulated signals so that interference from coloured media and foreign bodies can be avoided. The SFR readout unit is composed of nine optical modules for simultaneous measurement of both parameters. The system is suitable for use with shake flasks with 125, 250, 500, 1000 and 2000 ml volumes. The complete system can easily be mounted on the support of any standard shaker and hence fits into most shaking machines. Measurement data are transmitted wirelessly in real time. Because all system components are pre-calibrated there is no need for further on-site calibration.
Pilot Study with Yeast Cells
A pilot study on yeast cell cultures demonstrates the performance capabilities of the new measurement technique. The influence of different shaking frequencies and fill volumes on the oxygen content during the dynamic growth phase is of particular interest.
Dynamic Growth Phase
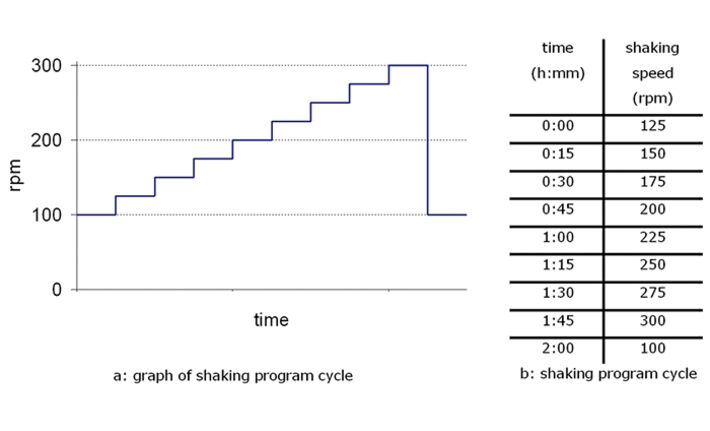
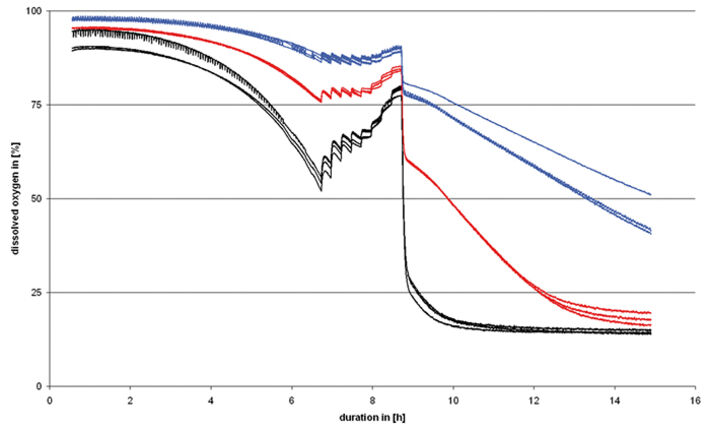
Changing the shaking frequency affects the dissolved oxygen (DO) content of the sample. A simple test set-up with stepwise increases in the shaking frequency over a period of two hours shows this clearly (Fig. 4). Starting at the base value of 100 rpm, the frequency is increased by 25 rpm every 15 minutes. After the maximum frequency of 300 rpm has been reached incubation is continued at 100 rpm. The shaking program started when the optical density gradient ΔOD reached 0.4. This ensured that the measurement series was in the region of exponential growth (Fig. 4). In order for the effect of the fill volume on the DO content to be determined in parallel, measurements were carried out on shake flasks (250 ml) with different fill volumes (12.5, 25 and 50 ml). The results for identical samples (n=3) were in good agreement. Each increase in shaking frequency (Fig. 3b) resulted in a considerable increase in the DO content. This was most pronounced in the samples with a fill volume of 50 ml and less so for lower fill volumes. After each increase in frequency the DO settled within a few minutes to a new higher level - however, only for a short time before dropping again. This can be attributed to the fact that a new equilibrium between the amount of oxygen input and the oxygen consumed by the culture was attained rapidly.
Improved Culture Process
The SFR system is an ideal tool for fast and non-contact determination of cell-specific oxygen metabolism. Reliable statements on changes in the DO of the culture over time can be made, even for long measurement series and high shaking frequencies. This applies in the same way to online measurement of the pH. For complicated culture tasks this monitoring function ensures maximum reliability and improves process efficiency. The pilot study carried out reveals the great potential and multitude of possible applications for online oxygen determination. The advantage lies in the fact that no system disturbances are needed for measurement. Thus, transfer of O2 to the cells is not interrupted. Transient anaerobic conditions can hence be avoided. Conclusions that can be used for further optimisation of the culture system - in terms of shaking frequency, sample volume, required culture duration, media composition, media changing times, optimum harvesting times and possibly also metabolically caused inhibition mechanisms in the culture through secondary products - can be drawn from the measurement data. The non-invasive measuring principle also has particular advantages for the culture of vertebrate cell lines. It reduces the risk of contamination. The SFR is an effective tool for low-cost development for new cell lines, reliable screening processes, media optimisation and preliminary tests within the framework of process scale-up.
1. Karin Benz and Jürgen Mollenhauer (2008) , Quality Assessment in 3D Cultures of Disc-Chondrocytes: Optosensoric Online Monitoring of Oxygen and pH Kinetics, BioProcess International Vol. 6 (11), pp. 54 - 59.




