Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Cellular Oxygen Consumption in Microfluidic Devices
Monitoring oxygen tensions with the VisiSens system
Christopher Ochs1, Junichi Kasuya2 and Andrea Pavesi1
1Singapore MIT Alliance for Research and Technology, BioSystems and Micromechanics, Singapore
2MechanoBiology Laboratory, Department of Biological Engineering, Massachusetts Institute of Technology, MA, US
Microfluidic devices fabricated from cyclic olefin copolymer (COC) and polydimethylsiloxane (PDMS) were combined with VisiSens sensor foils to enable monitoring of oxygen tensions in the devices. The oxygen consumption of rat lung microvascular endothelial cells (EC) and rat hepatocytes (Hep) was numerically simulated and experimentally validated using the VisiSens set-up. The VisiSens system enabled determining oxygen levels in the microfluidic devices and allows suggesting suitable culture device material depending on the application and cell line of interest. Furthermore, a previously characterized hypoxia microfluidic chip was evaluated and VisiSens was found to exhibit superior sensing capabilities when compared to similar products.
In recent years, microfluidic devices have been established as versatile platforms to mimic certain tissue microenvironments and to study cellular behavior and signaling. Due to their optical transparency, biocompatibility and ease of fabrication, devices based on polydimethylsiloxane (PDMS) are usually employed for lab-scale studies, but PDMS has disadvantageous characteristics in terms of adsorption of small hydrophobic species. Recent reports investigating the oxygen distribution and manipulation demonstrate growing interest in cell culture under hypoxic or anoxic conditions. Here, hard-plastic devices made of cyclic olefin copolymer (COC), polystyrene (PS), or polypropylene (PP) amongst others, offer an oxygen-impermeable alternative and are also easily mass-produced. However, little is known about the oxygen consumption of cells during culture inside the devices and the possible impact on cell viability / behavior. Monitoring of oxygen tensions inside microfluidic devices fabricated from COC and PDMS was performed with the VisiSens system in order to assess their suitability for cell culture. Furthermore, a previously published hypoxia device was evaluated using the VisiSens technology. The VisiSens detector unit (DU01, PreSens) was mounted vertically in a variable incubator and the microfluidic devices (bonded to sensor foils and filled with collagen gels and cells) were placed on top (Fig. 1). The oxygen consumption of the cells was monitored using the VisiSens software over several hours.
Materials & Methods
High-throughput COC and PDMS chips have been designed and fabricated, comprising a long gel region with adjoining, separated media channels. A novel hypoxic chip based on PDMS was also evaluated. This chip consists of a central gel region flanked by connected media channels, and peripheral gas channels, which are physically separated from the media channels by a thin oxygen permeable PDMS membrane. Direct bonding of pristine sensor foils (SF-RPSU4, PreSens) to the chips produced mechanically unstable constructs or badly sealed devices. It was decided to spin-coat a thin layer (30 µm) of PDMS onto sensor foils with removed protective coating, since PDMS is known to bind well to both PDMS and COC. The resulting PDMS-coated foils were then irreversibly bonded to activated COC and plasma treated PDMS chips with good sealing of the microfluidic channels. The functionality of the sensor foils was confirmed after PDMS coating, curing and plasma treatment and no significant difference in performance was observed when compared to pristine foils. To facilitate cell adhesion, devices were then coated with poly-D-lysine (1 mg/mL in dH20). A 2.5 mg/mL type-l collagen gel was introduced into the device gel region. Immediately prior to cell seeding, the media channels were filled with a 50 ng/mL aqueous solution of fibronectin for
4 h. Rat lung microvascular endothelial cells (EC) and rat hepatocytes (Hep) were used in the present study. Both cell types were seeded in the device media channels at a density of 4 M cell/mL and left to attach for 30 min. Unattached cells were then washed out with fresh media and devices were cultured for the indicated time periods in a variable incubator (5 % CO2, 37 °C). The oxygen consumption in the devices in the presence of EC and Hep cells was also simulated using commercial finite element software (COMSOL Multiphysics v4). All measurements were evaluated using the VisiSens AnalytiCal 1 software.
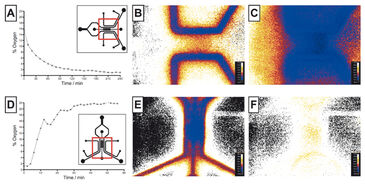
Oxygen Tensions in Hypoxia Chip
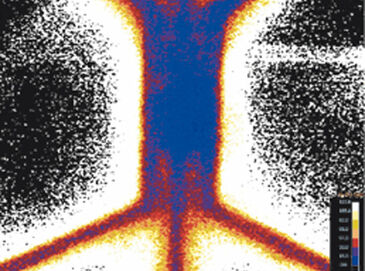
The novel microfluidic chip capable of establishing uniform or gradient hypoxic conditions over a central cell region was tested in order to compare the VisiSens technology to other existing oxygen sensors. The hypoxia chip was filled with collagen and water. On-chip calibration was achieved by introducing air-saturated and sulfite solutions into the gas channels temporarily. Subsequently, the gas channels were perfused with nitrogen gas to create uniform hypoxic conditions. The oxygen concentration was measured over time in the gel region (Fig. 2 B) and the oxygen tensions were found to decrease to 3 % within 1.5 h and to 1 % within 4 h. Compared to previous data, this result better matches the numerically simulation data (3 % after one hour), demonstrating the potential of the VisiSens system. Then the hypoxia chip was filled with oxygen-depleted (sulfite) solution and air was perfused through the gas channels to demonstrate the inverse effect. As shown in Figur 2 C, reoxygenation from 1 % hypoxia in the central gel region is complete after approx. 1 h (21 %). Gradients of oxygen could also be established and evaluated using VisiSens.
Cellular Oxygen Consumption
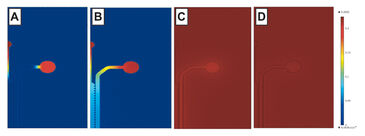
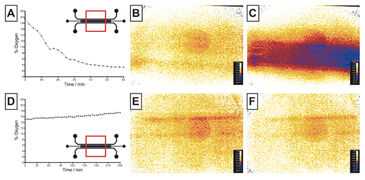
Prior to evaluation, the oxygen consumption of Hep and ECs was simulated in both COC and PDMS devices (Fig. 3). The same cell seeding density as in the experimental validation was used for modeling. The simulation results demonstrate that oxygen depletion is likely when Hep are cultured in COC (0% at 0.5 h), high consumption is expected for EC in COC (6 % at 2 h), and normal oxygen levels for Hep and EC in PDMS (21 %). Both cell lines were independently seeded in microfluidic devices and cultured over 2 days, and their oxygen consumption was monitored with the VisiSens system until steady state was established. Hepatocytes exhibited high oxygen consumption and depleted almost all oxygen within the impermeable COC device within 1 h (3 %, Fig. 4 A - C), which is comparable to the simulation results. In thin PDMS devices, however, oxygen can be replenished from the environment, resulting in stabilization of oxygen levels between 15 - 17 % over 4 h (Fig. 4 D - F). These results differ significantly from the simulated values, which may be attributed to conservative simulation parameters. These data suggest that COC devices are suitable for hypoxic cell culture of hepatocytes, whereas PDMS devices of defined thickness should be chosen if an intermediate range of oxygen levels is desired. For ECs, only moderate oxygen consumption was expected, which was confirmed by both simulation and experimental data obtained from cell culture in sensor foil covered devices. No significant difference was observed between COC (stable around 21 - 19 %) and PDMS (21 %) and both materials can thus be deemed suitable for normoxic culture of this cell line.
Conclusion
A previously characterized hypoxia microfluidic chip could be successfully evaluated and the VisiSens system exhibited superior sensing capabilities when compared to similar products. The biocompatibility and non-invasive operation mode of VisiSens sensor foils also enabled to determine oxygen levels in microfluidic cell cultures and suggest suitable device materials (COC or PDMS) depending on the application and cell line of interest. Microfluidic devices equipped with oxygen sensing capabilities are promising candidates for drug screening studies and hypoxic cell culture applications.