Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Identifying Water Oxidation Catalysts for Clean Fuel Production
High throughput screening of water oxidation reactions with OxoDishes®
R. J. Detz1, Z. Abiri1,2, A. M. Kluwer2, J. N. H. Reek1
1Van't Hoff Institute for Molecular Sciences, University of Amsterdam, NL
2InCatT B. V., Amsterdam, NL
The splitting of water in a light-driven reaction is one of the most promising processes for producing clean fuel. In order to create an efficient process suitable reaction catalysts must be found. In a high throughput screening we performed more than 200 reactions with different catalysts inside OxoDishes® and recorded the oxygen production. This way we were able to identify several catalysts that could form oxygen at the used reaction conditions. Comparison with Clark-electrode measurements confirmed results gathered with the OxoDishes®. The OxoDishes® proved to be a robust and versatile tool when screening for molecular water oxidation catalysts (WOCs).
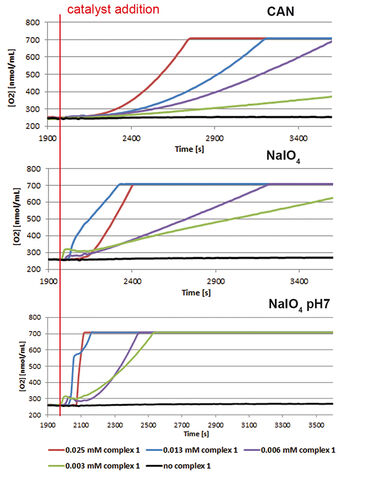
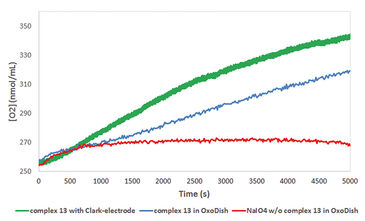
Two half reactions take place when splitting water in a photoelectrochemical (PEC) cell, water oxidation at the anode side and proton reduction at the cathode side. Both these reactions require their own specialized catalyst. In the search for new and more efficient catalysts high throughput techniques are applied. However, often it is difficult to find the right equipment, as most of it is not suitable for measuring the critical observable parameter of water oxidation, molecular oxygen. The Van´t Hoff Institute of Molecular Sciences of the University of Amsterdam in cooperation with InCatT B. V., a company specialized in high throughput catalyst research, developed a new screening application using the OxoDish® and SDR SensorDish® Reader technology of PreSens. After the sensitivity of the OxoDishes® was examined in initial tests with a known catalyst, a screening for WOCs was conducted. Several metal complexes were investigated, with a focus on iron-based water oxidation catalysts (in order to reduce catalyst costs). To confirm the data gathered with the OxoDishes®, oxygen measurements for one catalyst were repeated with a Clark-electrode.
Materials & Methods
The catalytic complexes were synthetized according to literature procedures [1, supporting information]. Typically, only one half-reaction - the anode side - of full water splitting is investigated. Therefore, an artificial electron (and proton) acceptor is needed to substitute for proton reduction at the cathode side. In this study cerium ammonium nitrate (CAN) as one-electron acceptor, and sodium periodate (NaIO4) as two-electron acceptor were applied. A 24-well OxoDish® was placed on the SDR SensorDish® Reader. Its wells were filled with typically 1 mL aqueous solution of 0.12 M chemical oxidant (CAN, NaIO4), and left for about 15 min for equilibration. Then the catalyst solution (typically 0.2 mL of 0.3 mM catalyst) dissolved in double-distilled water, and if required with some acetonitrile for dissolving the catalyst, was gently added. Wells filled with similar solutions, but without catalyst or oxidant served as a control. After the catalysts were added the plate was capped with a plastic seal to prevent gas diffusion. For comparison of OxoDish® data with a Clark-electrode a similar procedure was applied. After calibrating the electrode the chamber was filled with NaIO4-solution in phosphate buffer (1.67 mL, 0.06 M, pH 7); then the catalyst solution in water (0.33 mL, 0.5 mM) was added, and the chamber was capped.
Screening with the OxoDish®
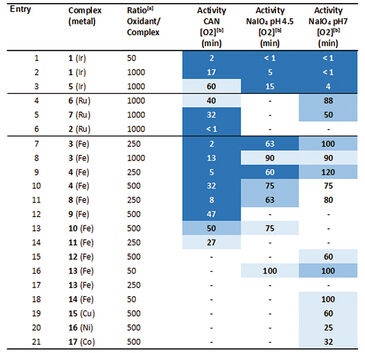
First the sensitivity of the OxoDishes® was examined with CAN at pH 0.7 and NaIO4 in aqueous buffered solutions of pH 4.5 and 7, together with complex 1 ([IrCp*(NHC)Cl2)], which is known to oxidize water with the two chemical oxidants. Oxygen kinetics recorded with the SDR are shown in Figure 1. In control wells containing only the buffered chemical oxidant solution and no catalyst no increase in oxygen levels was observable, while reaction wells containing the catalyst showed a steady increase in oxygen concentration. Oxygen levels rose either rapidly, reaching a maximum within a few measurement points, or slower, depending on the catalyst concentration used. At 700 nmol/mL oxygen concentration the upper limit of the OxoDish® measurement range was reached, and the graphs show constant values after this maximum detectable value was exceeded. Next we screened for new WOCs and performed over 200 reactions in OxoDishes®, examining various metal complexes (so far unknown for water oxidation catalysis) using different chemical oxidants and different pH (see Tab. 1). The screening showed that all tested Ir- and Ru-based complexes produced oxygen. The relative rate of oxygen allowed comparison of catalyst activity and showed that iridium catalyst 1 and ruthenium catalyst 2 are among the fastest WOCs so far. In order to find less expensive transition metal catalysts the screening particularly focused on iron-based WOCs. The iron catalysts 3 and 4 formed oxygen under applied reaction conditions, and the closely related complexes 8 to 11 were also active, especially when used with CAN. Besides analogues to benchmark WOCs also some novel leads were found, as e. g. Fe-EDTA complex 12 and Cu-EDTA complex 15, which demonstrated a low increase in oxygen reaching a maximum only after one hour reaction time. Dinuclear Fe-complexes 13 and 14 also showed active oxygen evolution using NaIO4 as chemical oxidant. Although those complexes are less active than benchmark Fe-complexes, the slow increase in the oxygen level indicates their good stability, which makes these complexes interesting for further optimization. For complex 13 we performed Clark-electrode measurements in order to confirm the water oxidation activity. The Clark- electrode experiments showed similar rates of oxygen formation like we observed in the OxoDishes® (see Fig. 2). The OxoDishes® can even be used to study the rate and thus kinetics of the reaction under certain conditions demonstrating the versatility of this parallel reaction and analysis tool.
Conclusion
Our test on the sensitivity and suitability of the Oxo- Dishes® and SDR SensorDish® Reader for WOC screening showed that this easy-to-use device is an ideal and versa- tile tool for parallel monitoring of oxygen kinetics in water oxidation reactions. More than 200 reactions could be investigated in this study, revealing promising new leads. These leads found with the screening are currently examined further with other techniques in order to describe the catalysts in more detail. Besides its already extensive catalyst research portfolio, InCatT B. V. is currently also developing parallel evaluation methods for oxygen- consuming catalysis using the PreSens OxoDishes®.
Application Note adapted from
R. J. Detz et al., ChemSusChem (2015) 8, 3057 - 3061: A Fluorescence-Based Screening Protocol for the Identification of Water Oxidation Catalysts


