Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
In vivo Monitoring of Haemolymph pCO2 in Dragonfly Nymphs to Determine the Resting State Value
Use of fiber optic pCO2 Microsensors to reliably measure in vivo haemolymph pCO2 in insects
Daniel J. Lee1, Fernando Martinez Ferreras2, Martin Gutbrod2 and Philip G. D. Matthews1
1University of British Columbia, Department of Zoology, British Columbia, Canada
2PreSens Precision Sensing GmbH, Regensburg, Germany
The objective of this study was to monitor the haemolymph pCO2 of water-breathing dragonfly nymphs in vivo. However, the small body size and haemolymph volume of these animals prohibited the use of standard in vitro measurement techniques and pCO2 electrodes. To overcome these issues, we used 250 μm diameter PreSens fiber optic pCO2 microsensors which could be implanted directly into the insect's haemocoel, providing continuous real-time monitoring of haemolymph pCO2. In combination with video monitoring of abdominal ventilation frequency, test results show that the haemolymph pCO2 of a resting dragonfly nymph fluctuates between 1 to 1.5 %, varying inversely with ventilation rate.
Water has approximately 30 times less available oxygen (O2) compared to air [1], and as a result water-breathing animals must ventilate large volumes of water to extract sufficient amounts of O2. However, as water is also a good sink for carbon dioxide (CO2), the high ventilation rate of water-breathers causes them to rapidly excrete their respiratory CO2, leading to a low internal pCO2. In comparison, air-breathers can readily satisfy their O2 demands and so ventilate slower, leading to a greater accumulation of internal CO2. This difference in internal CO2 levels between air- and water-breathers has been observed across a variety of water-breathing animal lineages that have evolved the ability to breathe air, including fish [1, 2], amphibians [3], and crustaceans [4]. This observation has led to the notion that low internal pCO2 is an unavoidable consequence of breathing water. However, due to the difficulties associated with measuring the pCO2 of small animals, there is no information regarding how the blood/gas values of developmentally amphibious insects change as they transition from breathing water to air during their life cycle. Measuring internal pCO2 in insects is not a simple task. It is difficult to obtain more than a few microliters of haemolymph due to their small body size, which prohibits using gas analysis methods that require larger blood volumes. The absence of cannulation techniques also complicates the measurement of physiologically relevant pCO2 for aquatic insects, as blood sampling can only occur once the animal has been removed from the water. However, pCO2 microsensors avoid all these problems by being small enough to be implanted directly into the haemocoel of aquatic insects for in vivo measurement of pCO2.
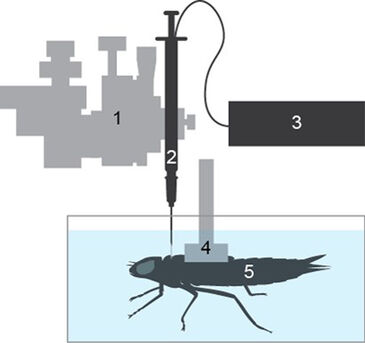
Materials & Methods
Monitoring of internal pCO2
Needle-mounted pCO2 microsensors were calibrated in 0.9 % NaCl solution bubbled with 0, 0.1, 1, 3 and 5 % CO2 in N2 by mixing a certified 5 % CO2 in N2 compressed gas mixture with N2 using two 0 - 500 mL min-1 mass flow controllers (MC-500SCCM-D/5M, Alicat, Arizona, USA). Water-breathing Anax junius dragonfly nymphs were captured from local ponds around the University of British Columbia Point Grey campus. They were individually housed in 2 L aquaria connected to a recirculating filter system under lab conditions (20 - 21 °C and 12:12 daylight cycle) for a minimum of two weeks. Prior to measurement, final instar nymphs were removed from their aquaria and their dorsal surfaces were quickly patted dry with paper towels. The dorsal wing-buds of the nymphs were then pressed into a concave plastic harness covered in polyvinylsiloxane dental impression material (President light body impression material, Coltène Whaledent, Switzerland) and allowed to cure. After solidification, the harness was attached to a 3-axis micromanipulator (M33301R, World Precision Instruments, USA) which was used to position the nymphs on the bottom of a 10 x 11 x 17 cm polypropylene container (Fig. 1) which was submerged in a temperature controlled water bath maintained at 21 °C. A second micromanipulator holding a 1 mL hypodermic syringe with a 20 gauge needle was used to make a small hole through the nymphs' cuticle in the middle of the thorax, between the pro- and meso-thoracic segments. The syringe was then replaced with a calibrated needle-mounted pCO2 Microsensor (PreSens, Germany), and approximately 1 mm of the fiber optic sensor tip was inserted into the hole. The hole and sensor were then covered in polyvinylsiloxane dental impression material to form a water-tight seal and left to cure. The polypropylene container was then filled with dechlorinated Vancouver tap water, continuously bubbled with room air, and covered with fine mesh to reduce the visual agitation of the nymphs. pCO2 readings were recorded once every five minutes using a pCO2 micro device (PreSens, Germany) and the pCO2 micro View software (PreSens, Germany).
Ventilation frequency
In order to compare the haemolymph pCO2 measurements to the insect's gas exchange behavior, we monitored ventilation frequency by tracking abdominal pumping using a B/W camera (Ikegami, Japan). The camera was connected to a desktop PC running EthoVision XT (EthoVision, USA) which recorded the change in abdominal area, and the data was analyzed by Labchart 8 (ADInstruments, Australia) to produce average breaths per minute during one hour intervals.
pCO2 Microsensor stability
To determine the stability of the pCO2 microsensor, they were tested in 0.9 % NaCl solutions equilibrated to 2 and 3 % gaseous CO2 in N2 immediately after calibration and again after 40 h of implantation in the dragonfly nymph.
Results
Monitoring of internal pCO2
Measurements were made on one nymph, and the data trace is shown in Fig. 2. By 20 hours the pCO2 had stabilized between 1 to 1.5 % and the ventilation frequency had declined to 10 BPM. Both the pCO2 and ventilation frequency then began to oscillate in an inverse manner (Fig. 2, 3). However, there appears to be a general trend of pCO2 stabilizing between 1 to 1.5 % whenever the ventilation frequency was within the normal 'resting' frequency of 5 to 10 BPM that has been measured previously in quiescent dragonfly nymphs. This finding suggests that dragonfly nymphs reach a resting pCO2 value of 1 - 1.5 % 20 hours after implantation. Furthermore, this shows that the pCO2 microsensors are capable of resolving changes in internal CO2 in response to changes in ventilation.
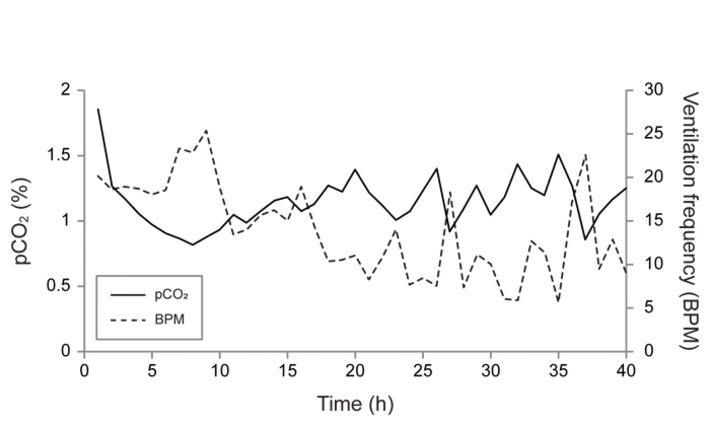
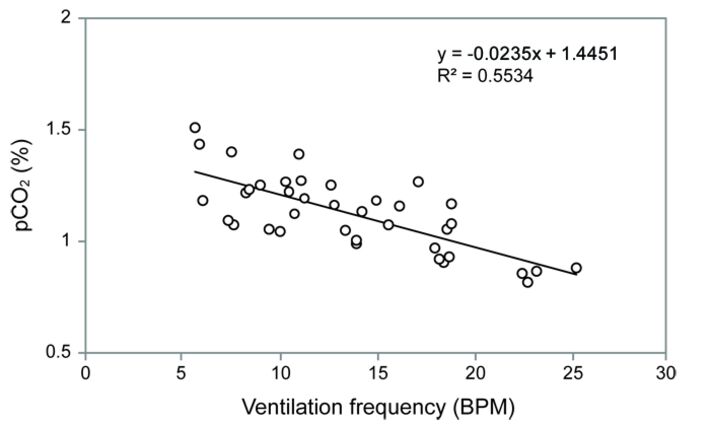
Testing of long-term stability
The implanted microsensor was recovered from the nymph intact, allowing the pCO2 readings before and after the implantation period to be compared (Fig. 4). As expected, the pCO2 readings were slightly higher following implantation. This was due to the slow photobleaching of the microsensor over the course of 866 sampling events. However, the probes were robust enough to be recovered from the nymph intact, and this small drift could be quantified. In the physiological environment of the insect's haemolymph, the probes remained stable. This demonstrates accurate pCO2 measurement by the microsensor during the experiment.
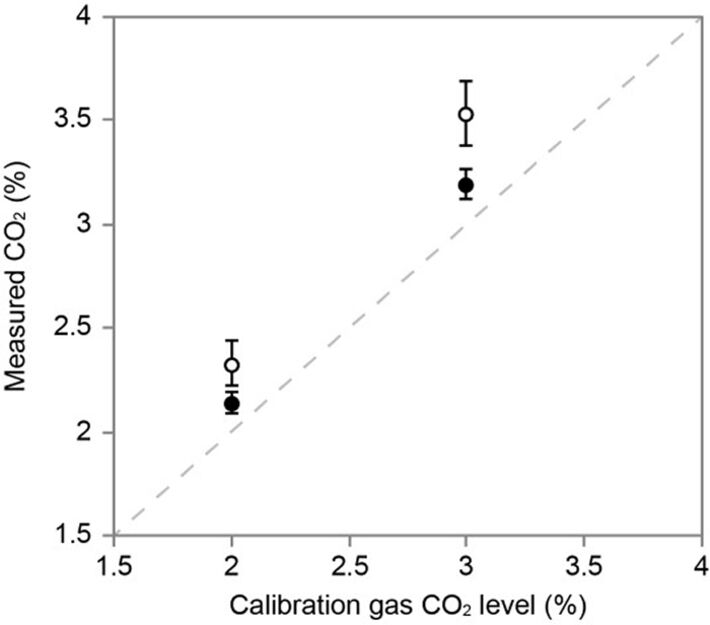
Conclusion
PreSens pCO2 microsensors allowed in vivo monitoring of haemolymph pCO2 in a small aquatic invertebrate, a task that is not possible using other conventional methods. As such, these microsensors would be ideal candidates for monitoring CO2 levels in other small biological systems restricted by small size.
References
[1] Ultsch, G. R. (1996). Palaeogeogr. Palaeocl. 123: 1 - 27
[2] Garey, W. G. and Rahn, H. (1970). Resp. Physiol. 9: 141 - 150
[3] Erasmus, B. W., Howell, B. J. and Rahn, H. (1970). Resp. Physiol. 11: 46 - 53
[4] Truchot, J. P. (1975). Resp. Physiol. 23: 351 - 360


