Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Insect Cell Cultivation in Orbitally Shaken Flasks with Sensors
Oxygen mass transfer in insect cell-based high cell density cultivations
N. Riesen, C. Ries, R. Eibl, D. Eibl
Zurich University of Applied Sciences, School of Life Sciences and Facility Managment, Institute of Biotechnology, Biochemical Engineering and Cell Cultivation Techniques, Wädenswil, Switzerland
Insect cell-based processes have become increasingly influential for tool protein and Virus-like Particle (VLP) vaccine productions over the last years. At small scale insect cells are normally grown in non-instrumented orbitally shaken disposable flasks, in most cases Erlenmeyers, in order to perform seed train productions and early stage process developments. The Shake Flask Reader with non-invasive optical sensors for pH and DO is an important contribution to easy quantification of oxygen mass transfer in Erlenmeyers recently made. In addition, this allows controlled processing at mL-scale as exemplified for described Sf-9 and Sf-21 cell mass propagation procedures.

Insect Cells and their Characteristics
Since the early 1970s the suitability of insect cells (in conjunction with the Baculovirus Expression Vector System, BEVS) for production of complex proteins used in structural analyses, functional studies, manufacture of diagnostics and vaccines have been described in numerous articles and reviews. The applied production cell lines (Sf-9, Sf-21 and BTI-TN-5B1-4, also known as High FiveTM or Trichoplusia ni cell line) derive from the Fall Armyworm (Spodoptera frugiperda) and the Cabbage Looper (Trichoplusia ni). Even in batch mode and at temperatures between 27 °C and 28 °C, and a pH value ranging between 6.2 and 6.9 these cells grow up to high cell densities (>1 x 107 cells mL-1). For this it is of course necessary that an optimized serum-free culture medium exists, which supports cell growth as well as a qualified cultivation system preventing shear stress damage and guaranteeing sufficient oxygen supply. The parameter characterizing the oxygen transfer in a cultivation system is the kLa value (also known as oxygen transfer coefficient).
Determination of kLa Values
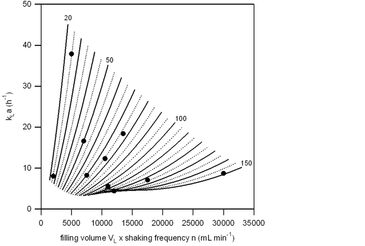
In order to determine kLa values for shaken flasks from PreSens (250 mL total volume, standard vented caps), their coupling with PreSens' SFR (www.presens.de/SFR) is required. The SFR unit mounted on Infors' shaking incubator Ecotron (25 mm shaking diameter) has nine double optical modules which enable wireless real-time monitoring and display of pH and DO. By using the well-known standard gassing-out method (see Fig. 1), nine different experiments (statistically designed with STAVEX 5.0 software) at varying filling volumes (between 25 - 150 mL) as well as shaking frequencies (between 80 - 200 rpm) were carried out. All flasks were running with Sf-900 III SFM from Gibco Invitrogen at a temperature of 27 °C. DO concentrations were monitored over the time and experimental as well as STAVEX 5.0 model derived kLa values were calculated as described in detail by Ries et al. [1]. At selected cultivation parameters experimental kLa values ranged between 4.4 and 37.9 per hour; these values correspond well with the modeled oxygen transfer data (see Fig. 2).
Oxygen Transfer Studies during Mass Propagation
Mass propagations of Sf-9 and Sf-21 cells were performed in 250 mL shake flasks equipped with integrated and pre-calibrated pH and DO sensors from PreSens. They lasted between seven and eight days while the flasks were filled with 100 mL Sf-900 III SFM, inoculated with 5 x 105 cells mL-1 and incubated at 27 °C and 110 rpm. Under these conditions an oxygen transfer coefficient of 5.6 per hour can be assumed. Sampling was done in two different ways. In experiment series 1 (Sf-9 cell mass propagation) each day of cultivation a sample of all flasks was taken, whereas in experiment series 2 (Sf-21 cell mass propagation) a sample of only one shake flask was taken per day. The sample analyses included measurements of living cell density and viability (with NucleoCounter from Chemometec and Cedex HiRes from Innovatis), and off-line determinations of pH, glucose, lactate, ammonium, glutamine, glutamate (with BioProfile 100 from Nova Biomedical).
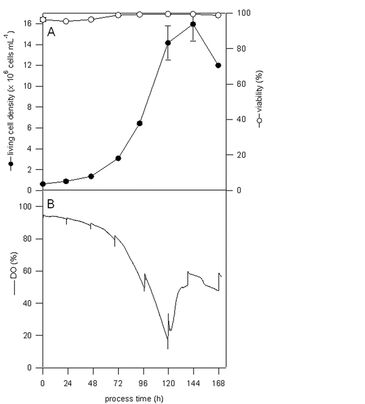
Fig. 3 shows a DO course which is influenced by sample taking respectively opening the sterile closure such as observed in experiment series 1, where a maximum living cell density of 1.6 x 107 cells in mL-1 was reached at day six of cultivation. However, no clear statement on possible mass transfer limitations can be made.
From results of experiment series 2 in which a non-influenced DO course could be guaranteed it is obvious that cell growth of Sf-21 suspension cells stops below oxygen saturation of 10 % [1]. Because of the fact that this observation coincides with those of further groups and growth inhibitions resulting from culture medium appear implausible to our experience, we suppose an oxygen limitation during day five of cultivation. Interestingly, this is the time immediately before achieving the maximum living cell density, which was again 1.6 x 107 cell mL-1. Independent on the experiment series, all on-line pH data corresponded well with those of off-line measured samples.
Conclusion
Our results reveal PreSens' shake flasks, allowing non-invasive pH and DO measurement, advantageous usage for process characterization and optimized processing in case of insect cell cultivations at small scale. Oxygen is the key element for high cell density growth aimed in seed train productions and high product titers desired in protein productions.
[1] Ries C, John G, John C, Eibl R, Eibl D (2010), A shaken disposable bioreactor system for controlled insect cell cultivations at millilitre-scale, Life Science Engineering
Vol. 10 (1), pp. 75 - 79