Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
kLa Measurement in TubeSpin Bioreactors
T. Backoff1, J. Malig1, S. Werner2, G. T. John3, D. Eibl2
1Anhalt University of Applied Sciences, Department 7 - Applied Biosciences and Process Engineering, Köthen, Germany
2Zurich University of Applied Sciences, School of Life Sciences and Facility Management, Institute of Biotechnology, Wädenswil, Switzerland
3PreSens Precision Sensing GmbH, Regensburg, Germany
TubeSpin bioreactors are used for cell cultivation as a cost effective alternative to larger scale cultivation systems. However, the characteristics of the tubes with regard to oxygen transfer have hardly been investigated. Using a specially adapted SFR Shake Flask Reader and chemical optical sensors with rapid response time from PreSens, kLa for TubeSpin systems was determined. The characteristics of these cultivation vessels were investigated more closely, which represents an important step towards defined screening processes by monitoring oxygen in TubeSpin bioreactors.
TubeSpin bioreactors are used for cell cultivation at mL scale in orbitally shaken incubators. Thus, development of optimized cell culture media and identification of specific cell lines can be carried out quickly and inexpensively [1]. There is currently very little information in the literature about oxygen transfer in TubeSpin bioreactors. Zhang [2] studied the characteristics of TubeSpin bioreactors and listed kLa values up to 29 h-1. The volumetric oxygen mass transfer coefficient (kLa) indicates the ability of oxygen transfer from gas phase to the liquid phase [3].
The SFR Shake Flask Reader
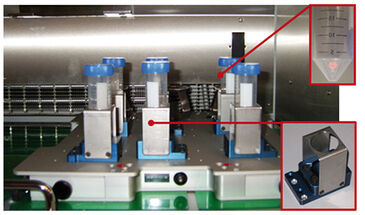
The PreSens SFR Shake Flask Reader is commercially available since 2009. It allows dissolved oxygen (DO) measurement in shake flasks with integrated disposable sensors, which are read out by a special reader unit mounted inside the shaker. The recorded data is transferred to a PC via wireless Bluetooth connection. PreSens further developed and extended this system so it can be used with TubeSpin bioreactors. The extensions comprise special adapters to hold the tubes and read DO values by means of the integrated disposable sensor spots (Fig. 1). DO measurement in TubeSpin bioreactors can be applied for monitoring screening processes with regard to oxygen consumption of the cells. Furthermore, it is now possible to characterize TubeSpin bioreactors more precisely by measuring the kLa values.
Optical Oxygen Measurement
DO measurements are conducted with chemical optical sensors attached to the bottom of the bioreactors (Fig. 1). A major advantage compared to conventional oxygen probes (e. g. Clark-type electrodes) is the miniaturized size of the sensor spots. The integration of larger conventional oxygen probes would change the flow behavior of the liquid inside the TubeSpin bioreactors. Furthermore, TubeSpin bioreactors are too small for conventional probes, and due to the orbital shaking movement liquid is not always in contact with the probe. A further advantage of the sensor spots is their response time of 10 - 30 s, which outranges conventional oxygen sensors. In 2002 such spots were already being used to determine kLa in shake flasks, and reliable values of up to 150 h-1 could be measured.
Medium, Methods & Equipment
Oxygen transfer rate (OTR) is measured at 25 °C in a coalescence reduced aqueous solution (0.2 M Na2SO4) using the dynamic sulfite method [4]. When Co2+ is present, sulfite reacts to sulfate consuming oxygen (chemical oxidation) until DO reaches the 0 % value (oxygen depletion). From that point on, DO is recorded using defined parameters (filling volume, frequency, amplitude) at intervals of 10 s until 95 % saturation is regained. kLa values are calculated from the measured DO by linearization of the response in a range from 20 % to 80 % of saturation concentration (oxygen saturation being realized by diffusion from ambient air using vented caps). kLa in TubeSpin bioreactors was measured in triplet with varying volumes (10 - 30 mL) and shaking frequencies (150 - 300 rpm). Furthermore, amplitudes of 25 and 50 mm, which are both common to laboratory shakers, were investigated. A statistical Design of Experiment (DoE) was used to determine the number of experiments and evaluate data.
Results
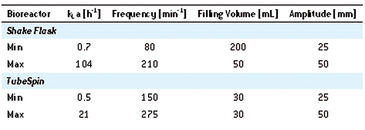
kLa values in a range of 0.5 h-1 ± 30 % up to 21 h-1 ± 10 % were determined in the TubeSpin bioreactors (Tab. 1). In single-use shake flasks, kLa values of up to 104 h-1 were determined. After evaluation of data, the following correlation of filling volume (FS), frequency (FR), and amplitude (AM) in TubeSpin bioreactors was identified using statistics software Visual XSel 11.0:
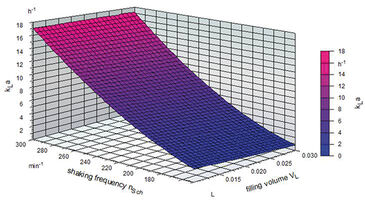
kLa = (1.196007 + 4.089 x 10-3 x FR - 80,2998 x FS - 0.04206 x AM + 0.166267 x FR x FS + 2.53 x 10-4 x FR x AM + 0.633247 x FS x AM)2
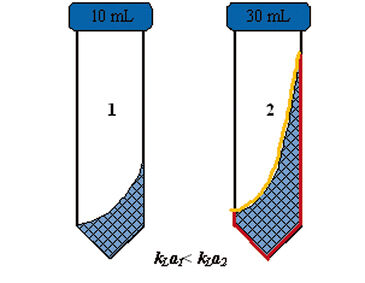
This correlation can be displayed in a three-dimensional surface plot (Fig. 2). The highest kLa values were achieved with an amplitude of 50 mm. Interestingly, experiments showed that high filling volumes with an amplitude setting of 50 mm and a high shaking frequency (> 250 rpm) resulted in high kLa values in the TubeSpin bioreactors. This behavior may be caused by an increased liquid-to-air-surface area and a larger area where the liquid is in contact with the vessel wall, which is supported by the cylindrical shape of the tubes. The liquid rotates as a thin film along the vessel wall, which may increase gas mass transfer into the liquid (Fig. 3). In shake flasks such an effect cannot be observed as fluid rotation is only possible in the lower part of the bioreactor due to the conical shape of the vessels [5]. These effects cannot be predicted by the statistical model at present, and further tests need to be conducted. With a kLa value of 21 h-1, CHO cells with a specific oxygen consumption rate of 9 x 10-12 g (cell h)-1 can be grown to achieve cell densities of more than 56 x 106 cells mL-1 under non limiting conditions.
Conclusion
With the SFR Shake Flask Reader DO measurements can be conducted faster and more efficiently than with conventional invasive sensor probes. The relatively free choice of measurement frequencies, and the fast response time of the disposable sensors enable stable DO measurement. With the miniaturized sensor spots, culture conditions can be monitored and adjusted as necessary even in small, simple bioreactors such as TubeSpin systems. Using the SFR, these "Black-Box" bioreactors are more accessible with regard to oxygen transfer investigations. This is an important step towards defined screening processes. A statistical correlation of motion parameters and the specific oxygen mass transfer coefficient kLa in TubeSpin bioreactors was determined using this system.
References
[1] Tsai, et al.: Noninvasive Optical Sensor Technology in Shake Flasks, BioProcess International, Vol. 10, No. 1 (2012), 50 - 56
[2] Zhang, et al.: Efficient oxygen transfer by surface aeration in shaken cylindrical containers for mammalian cell cultivation at volumetric scales up to 1000 L, Biochem Eng J 45 (2009), 41 - 47
[3] Schumpe, et al.: Gas Solubilities in Microbial Culture Media, (2008) 1 - 38
[4] Garcia-Ochoa, et al.: Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview, Biotechnolgy Advances 27 (2009), 153 - 176
[5] Maier, et al.: Advances in understanding and modeling the gas-liquid mass transfer in shake flasks, Biochem Eng J 17 (2004), 155 - 167