Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Light vs. Dark Oxygen Demand of Plankton Samples
O2 Measurements with SensorVials and the SDR SensorDish® Reader
Dr Sabine Schultes
Aquatic Ecology Group, LMU Munich, Germany
The dynamics of production and consumption of O2 in natural plankton communities change along environmental gradients and yield important information on the community ecology and biogeochemical cycling in the pelagic ecosystem. A dominant contributor to O2 production is the nano- and microphytoplankton, whereas oxygen is consumed by bacteria, phytoplankton, heterotrophic protists (e. g. ciliates) and mesozooplankton (e. g. crustaceans, rotifers, hydrozoans or ctenophores).
In these experiments, we tested SensorVials (2 and 4 mL format) and the SDR SensorDish® Reader by PreSens for respiration rate and photosynthesis measurements of natural plankton communities in light and dark conditions. The SensorVials have an integrated optical oxygen sensor at the bottom, which is read out with the 24-channel reader. This way multiple samples could be monitored in parallel.
Material & Methods
Plankton communities were sampled from the pond at LMU Munich, from Lake Brunnsee, Seeon, Germany, and from a coastal lagoon at Sletvik field station, Norway. Water samples were incubated in climate chambers with constant, near ambient temperature. PSt5 SensorVials (PreSens GmbH, Germany) of 2 mL and 4 mL volume were filled with water containing natural plankton communities. For one experiment, gelatinous zooplankton organisms were incubated in sterile filtered (0.2 μm) seawater. As a control, at least two vials per SDR were run with sterile filtered seawater. The control incubations yielded a stable and reliable signal for correction of the measured samples. Vials were placed inside an empty 24-well plate which was put on the SensorDish® Reader (PreSens GmbH, Germany) and changes in oxygen concentration were recorded every three to five minutes for several hours (see experimental details). For measurements in the light, the vials were placed in a black mask (SDR-MSV24, PreSens GmbH) instead of the well plate to avoid disturbance of the SDR optics due to the light. Vials for the dark measurement were placed in a normal 24-well plate and the plate and SDR were covered with aluminum foil.
Light / Dark Measurements of Lake Water with & without Black Shielding Mask
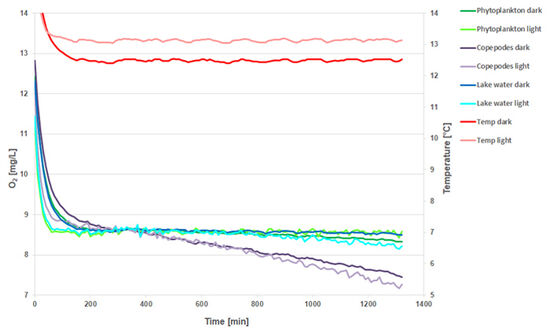
The first measurement was made with 4 mL Sensor Vials positioned in a transparent 24-well plate. For better comparison, a one-point adjustment was made at 320 min. The signal of the O2 sensor spots in the light (Fig. 2, light colors) is very 'noisy' in comparison to the dark measurements (Fig. 2, dark colors). The phytoplankton kinetics show more respiration in the dark than in the light conditions. However, for lake water, it is the other way around. The differences in the 6 replicates (data not shown) make it difficult to recognize tendencies. A clear reduction in O2 concentration is only detected in the copepode samples.
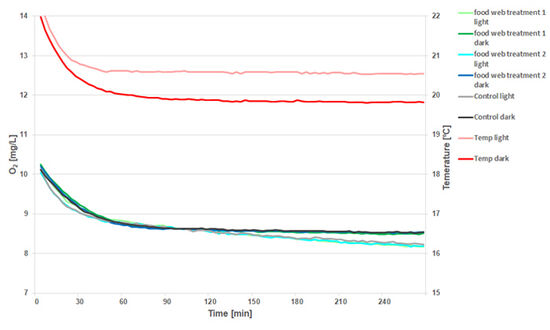
The second experiment was conducted using a black mask, the SDR-MSV24 provided by PreSens, which was placed on the SDR reader for the light incubation. In addition, new 2 mL vials with sensor spots were used.
The decrease of O2 concentration with time can be detected in the light and in the dark (Fig. 3). Different food web treatments did not cause any differences in respiration.
Again a one-point adjustment was performed at 120 min for better comparison of the slopes. The signal from the sensor spots in the light is comparable to the signal from the sensor reading in the dark regarding the noise ratio. The black shielding mask therefore efficiently shields the SDR optics from light so the sensors can be read out without disturbance. It was used for all further measurements in light conditions.
The detailed data analysis shows that the decrease in O2 concentration is different in the light compared to the dark measurement (Fig. 3). The oxygen in the light is being respired more rapidly than in the dark. This is unexpected behavior, since the respiration of heterotrophic plankton should at least to some extent be compensated by the production of O2 in the light due to the photosynthetic activity of the phytoplankton.
The faster rate of O2 decrease in the light can probably be explained by differences in temperature, that were observed between the two SDRs that were used. The SDR used for the light incubation was 0.8 °C warmer than the SDR in the dark (Fig. 3). The experiment was conducted in a climate cabinet with stable temperature. However, the fluorescent tubes used for illumination produce heat and were placed at close proximity to the vials and the reader. Alternatively, light inhibition of the natural phytoplankton communities cannot be ruled out.
Light / Dark Measurement of O2 Consumption in Marine Plankton Communities Using 4 mL SensorVials
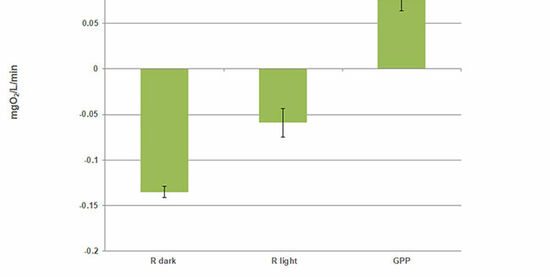
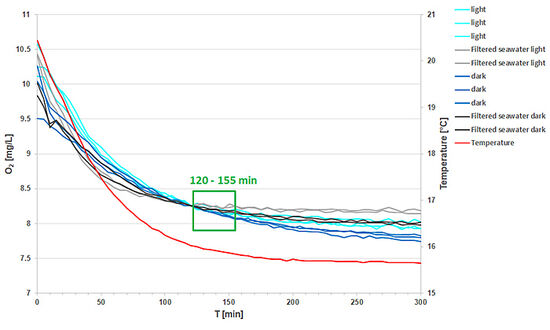
In this experiment, the changes of O2 concentration in marine plankton communities were measured in the light and in the dark, using the 4 mL SensorVials. The time courses of the concentration change in the light yielded less negative slopes than for the communities incubated in the dark (Fig. 4). Slopes were corrected for the change in O2 concentration measured in 0.2 μm filtered seawater. From the differences in light and dark respiration, gross primary production could be calculated (GPP = |Rdark | + Rlight). The ratio of GPP / Rdark indicates the presence of a net heterotrophic system.
Concerning the use of the SensorVials for this type of measurement, it has to be concluded that the changes in O2 concentrations are very small. To be able to detect such small changes, an exact match of the temperature in both SDRs is necessary. Though it was difficult to achieve, great care was taken to ensure both SDRs were incubated at the same temperatures.
The slopes used for calculation of respiration data in Fig. 4 were extracted with the SDR software over a short period (approx. 35 min) with measurements taken every 5 min. A portion of the curve was chosen, where both SDRs showed equal temperature, and where temperature changes were minor, e. g. a slow decrease from 16.2 °C to 15.8 °C. For this experiment, this was the time from 120 to 155 min. The kinetics are shown in Fig. 5. For better comparison of the slopes, a one-point adjustment was made at 100 min.
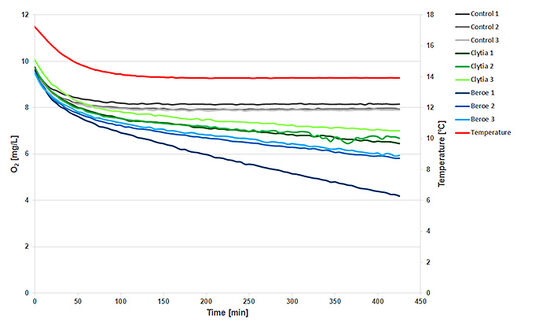
Respiration Measurement with Gelatinous Zooplankton in 2 mL SensorVials
For comparison, I also report the results of a respiration measurement with individuals of the genus Clytia sp. and Beroe sp.. The change in O2 concentration (Fig. 6) was clearly detected compared to the control (0.2 μm filtered seawater).The controls (grey lines) show a constant signal as soon as the temperature (red line) is constant. The 2 replicates of Clytia sp. (green) show very similar respiration, whereas one of the 3 replicates of Beroe sp. (blue) is more active than the other two.
Conclusion
Overcoming the difficulties to achieve an exact temperature match for several SDR plates, the use of PSt5 SensorVials can be recommended for both, the measurement of light and dark respiration rates from natural plankton communities and zooplankton. The black mask effectively shields the SDR optics from the surrounding light and allows recording of a clear sensor signal.
Both, the 4 mL and the 2 mL vials can be used for the whole community measurement. For the measurement of zooplankton the vial size should be adapted according to the size of the organism tested. We could not detect a negative effect of the small (2 mL) vials on the gelatinous zooplankton of up to 1 cm. Earlier experiments by colleagues (data not shown) with the 4 mL vials also yielded good results for copepods and cladocerans. Experiments conducted with rotifers at LMU Munich also show promising results with the use of 4 mL SensorVials. However, the use of 2 mL SensorVials may be preferable when working with very small mesozooplankton.