Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Localized Oxygen Depletion by Transmigrating Neutrophils Influences Inflammatory Resolution
Oxygen monitoring in cell-cell cross talk experiments with the SDR SensorDish® Reader
Eric L. Campbell, and Sean Colgan
Mucosal Inflammation Program, University of Colorado, Anschutz Medical Campus, USA
Recent research on intestinal inflammation has shown that mucosal metabolism influences disease outcome. Intestinal inflammation is accompanied by accumulation of neutrophils (Polymorphonuclear leukocytes = PMNs). Here we investigated the influence of transmigrating PMNs on O2 metabolis of the surrounding mucosa. Oxygen monitoring in cell-cell cross talk experiments was performed in OxoDishes® with the SDR SensorDish® Reader. Our findings showed how PMNs rapidly deplete local molecular oxygen to such an extent that epithelial cells in close proximity stabilize the HIF (hypoxia-inducible factor) transcriptional machinery. Moreover, we demonstrate how such localized O2 depletion is critical for inflammatory resolution.
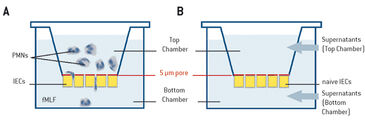
Transmigration of neutrophils to sites of infection or injury is necessary for the body´s own defense. Accumulation of PMNs during intestinal inflammation, however, can lead to chronic inflammatory states. Infiltration of the infected tissue by PMNs is accompanied by energy-demanding processes, such as e. g. migration, phagocytosis, and generation of an NADPH oxidase burst, which are thought to cause metabolic changes in the tissue during inflammation. In this context PMNs might have critical influence on the surrounding cells, but still very little is known about how such changes affect tissue function and disease outcome. In these experiments we analyzed how PMNs shape the tissue microenvironment by depleting local molecular oxygen, and whether this influences the transcriptional profile of the cells. We developed an experimental set-up to analyze cell-cell cross talk in a direct and indirect model (Fig. 1). For monitoring oxygen consumption of activated PMNs we applied the SDR SensorDish® Reader by PreSens with 24-well OxoDishes®, multi dishes with integrated oxygen sensors. Online measurements with the SDR are a great advantage in our experiments, as it allows high-throughput analysis. Using this model we were able to show how activated PMNs rapidly deplete local molecular oxygen and how this influences surrounding epithelial cells. In vivo tests (not described here, see [1]) furthermore revealed how such localized O2 depletion contributes to effective mucosal protection and inflammatory resolution.
Materials & Methods
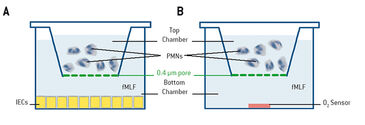
Neutrophils were examined during direct transmigration, indirect transmigration, coculture, or in real-time oxygen monitoring experiments with the SDR system. For direct transmigration, T84 intestinal epithelial cells (IECs) were grown on the underside of collagen-coated Transwell permeable supports (5.0 µm pore; Corning). Neutrophils (1 x 106) were applied to the top chamber, while in the bottom chamber a chemotactic agent (1 µm fMLF = N-formyl-methionine-leucine-phenylalanine in HBSS = Hanks´ Balanced Salt Solution) was added, and the neutrophils were allowed to transmigrate (Fig. 1 A). In the indirect model, conditioned supernatants from direct transmigration, as described before, were collected. Supernatants were clarified by centrifugation (350 x g for 10 min) and then applied to naive T84 monolayers (Fig. 1 B). In the cocultured model, epithelial cells were culture in 24-well plates (Fig. 2A) with PMNs suspended above the T84 cells on permeable supports (0.4 µm pore; Corning) that prevented physical interaction between PMNs and IECs. Real-time oxygen monitoring was performed with 24-well OxoDishes® on the SDR SensorDish® Reader (PreSens) in normoxia. OxoDishes® were pre-equilibrated with HBSS+ or HBSS+ containing either fMLF or PMA (phorbol myristate acetate = a tumor promotor). For patient PMN fMLF was used and for mouse PMN PMA was used as chemotactic. The transwell filters separated PMNs from the sensor (Fig. 2 B). The whole system was agitated to disrupt the unstirred liquid beneath the Transwell filter. Freshly isolated neutrophils from either whole venous blood of healthy volunteers or mouse bone-marrow-derived PMNs were applied to the filter. PMNs of wild-type (C57BL/6) mice and CGD (Nox2-/-) mice (Jackson Laboratories) were examined with the SDR. CGD mice phenotypically show symptoms of human chronic granulomatous disease (CGD) because they are lacking functional NADPH oxidase in PMNs which causes a deficiency in PMN respiratory burst activity. Microarray and analyses were performed as described in [1].
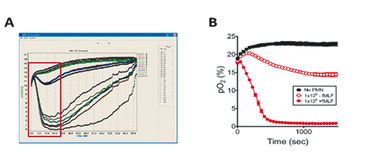
Influence of PMNs on the Microenvironment
Our initial experiments focused on the impact of PMN transmigration on the transcriptional profile of intestinal epithelial cells. Therefore, we conducted parallel experiments of the direct and indirect transmigration model for 90 min, harvested RNA after 2 h, and analyzed transcriptional profiles by microarray. Profiling in the direct model revealed mainly PMN-specific genes, which was caused by contamination of the monolayer with incompletely migrated neutrophils. Analysis of the indirect model showed that genes regulated as a result of factors released during transmigration were present. A number of hypoxia-regulated genes that were induced in both models in response to PMNs could be identified. Due to these findings we wanted to measure oxygen consumption of activated PMNs and determine the impact of PMN oxygen consumption on the transcriptional profile of IECs. We used 24-well OxoDishes® where PMNs were suspended above the oxygen sensor. Measurements showed clearly how PMNs rapidly depleted all available O2 within minutes dependent on cell number and activation factor. To ensure this O2 depletion was sufficient for epithelial cell in close proximity to sense hypoxia we performed coculture of PMNs suspended in a 0.4 µm pore Transwell above T84 intestinal epithelial cells in the presence of hypoxia-dependent adduct forming compound (pimonidazole-HCl) dye and activated with fMLF. After 0 min and 60 min cells were fixed and localized for hypoxia adducts with Hypoxyprobe-1 antibody (Hypoxyprobe). Nuclear extracts revealed that PMN activation induced stabilization of HIF at 3 and 6 h of exposure. For further examinations on mechanisms of PMN oxygen consumption and the contribution of PMN respiratory burst in O2 depletion, we harvested bone marrow PMNs from both wild-type C57BL/6 and CGD mice, and incubated the neutrophils with or without DPI (diphenyleneiodonium = NADPH oxidase inhibitor) for 15 min on ice. Then we monitored the PMNs´ O2 consumption with the SDR system. Activated PMNs of wild-type mice without DPI again rapidly depleted all available oxygen, while in presence of DPI oxygen consumption by wild-type cells was abolished. PMNs of CGD mice hardly consume any oxygen whether activated with PMA or not, in the presence or absence of DPI. Taking together all the data we collected during in vitro tests, results show that PMNs deplete sufficient O2 via NADPH oxidase burst, that nearby epithelial cells are able to sense hypoxia, and become transcriptionally imprinted.
Conclusion
The experimental set-up we used proved most suitable to investigate cell-cell cross talk and could be applied to other cell type models as well. Real-time measurements with the SDR SensorDish® Reader allowed high throughput analysis with continuous oxygen measurements and gave excellent insight in PMN oxygen consumption rates. These results contributed fundamentally to our findings that epithelial HIF stabilization in response to rapid O2 depletion by PMNs is critical for transcriptional induction of numerous protective genes. Further in vivo tests in mice demonstrated how localized O2 depletion by PMNs is critical for inflammatory resolution [1]. Our findings provide important insight into therapeutic options for inflammatory mucosal diseases.
Application Note adapted from:
[1] E. L. Campbell, et al.: Transmigrating Neutrophils Shape the Mucosal Microenvironment through Localized Oxygen Depletion to Influence Resolution of Inflammation; Immunity 40, pp. 66 - 77, 2014


