Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Measuring Dissolved O2 in Dimethyl Sulfoxide Solution in the Presence of Different Lithium Salts
Investigations on organic electrolytes with the OXY-1 SMA and optical O2 Sensor Spots
Jonas Lindberg and Balázs Endrodi
KTH - The Royal Institute of Technology, Chemical Engineering and Technology, Stockholm, Sweden
In Li-O2 batteries the concentration of dissolved oxygen in the organic electrolyte is a very important parameter. As being a reactant in the reduction reaction, its concentration has a direct effect on the reaction rate. So far, several studies have been reported on using different electrolytes or solvents for Li-O2 batteries. However, it is very difficult to compare the results gathered for these very different cases as the battery performance and stability is governed by multiple parameters. To deconvolute the effect of the different oxygen concentrations in these cases, the dissolved oxygen concentration must be measured reliably.
Measuring O2 Concentration in DMSO & DMSO Based Electrolyte Solutions
The dissolved oxygen (DO) concentration in anhydrous dimethyl sulfoxide (DMSO) and DMSO based solutions of different Li-salts was measured using the PreSens OXY-1 SMA and an optical oxygen sensor spot SP-PSt3. The sensor spot was immobilized on the inner wall of a simple glass vessel (Fig. 1). The vessel was subsequently filled with DMSO, which was kept in it overnight. We note, that this step was crucial in order to get reliable and reproducible results, which can be most probably related to the soaking and swelling of the sensor spot. The solutions were one after another transferred to this vessel and were vigorously bubbled with synthetic air (21:78 O2:N2 ratio, 1 bar) for 30 min before the measurement. After pointing the optical fiber connected to the OXY-1 SMA to the sensor spot, the oxygen concentration was measured, until a stable reading could be seen in the software (typically in a few minutes).
Sensor Evaluation
The sensitivity and calibration of the sensor was evaluated by measuring the O2 concentration in O2 saturated distilled water, which showed a good agreement with the literature value. In the case of DMSO, the first experiments aimed the testing of the measurement device's sensitivity to the dissolved oxygen concentration. Anhydrous DMSO was saturated with synthetic air and the oxygen concentration was measured. Similarly, the same solvent was saturated with pure oxygen, and the oxygen concentration was measured again. The measured data showed a clear, linear dependence on the dissolved oxygen concentration, agreeing with the results found earlier with a mass spectrometric measurement [1].
O2 Concentrations in Different Electrolyte Solutions
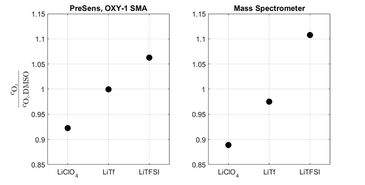
As the sensor was calibrated in aqueous solutions, the measured values could not be directly accepted, necessitating to recalibrate the sensor in DMSO. The results shown here are therefore presented as relative concentrations, always related to the measured oxygen concentration in the neat solvent (Fig. 2). As shown by these measurements, the oxygen solubility is not significantly affected by the presence of Li-triflate (LiTF), it increases with the addition of Lithium bistrifluoromethanesulfonimidate (Li-TFSI) to the solution, while it decreases when LiCLO4 is added as electrolyte. Importantly, these latter two are the most frequently used electrolytes in DMSO containing Li-O2 batteries. The oxygen concentration - which directly affects the chemistry of these batteries - is significantly different when using one of these salts instead of the other. Comparing results gathered with the same salt concentration, but not taking into account the different oxygen concentrations can therefore be very misleading in such studies.
Similar measurements were performed by applying a mass spectrometric method. Not going into the details, we can conclude that the two significantly different methods led to the exact same conclusions.
Reference
[1] Lindberg, J., Wickman, B., Behm, M., Cornell, A., Lindbergh, G., 2017. The effect of O2 concentration on the reaction mechanism in Li-O2 batteries. J. Electroanal. Chem. 797, 1 - 7