Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Monitoring Oxygen in Perfused Muscular Tissue Using Flow-Through Cells FTC-PSt3
K. Präbst1, A. Dragu2, C. Taeger2, B. Sommerfeldt1, H. Hübner1, R. Buchholz1, R. E. Horch2
1Institute of Bioprocess Engineering, FAU Erlangen-Nuremberg, Germany
2Department of Plastic & Hand Surgery, Univ. Hospital, FAU Erlangen-Nuremberg, Germany
Muscular tissue is strongly sensitive to ischemic conditions. This limits the time available for transplanting free muscular tissue strongly. Free flap transfer includes the separation of the muscle´s blood vessels and reattaching the major vessels in the recipient bed to the native blood supply. Between harvesting and anastomosis of the flap´s vessels, the tissue is exposed to ischemia. Transgressing the critical ischemic time can lead to apoptosis and even necrosing of the tissue, often ultimately resulting in partial or total flap loss. For bridging time during intraoperative complications which makes banking the free flap necessary an extracorporeal perfusion system is tested to keep the tissue alive and replantable. Oxygen as a critical parameter is monitored online during perfusion experiments using flow-through cells FTC-PSt3 connected to a multi-channel oxygen meter (OXY-4 mini) by PreSens. Results indicate the active consumption of total oxygen provided by the perfusate in crystalloid solutions making an active oxygenation unit implemented in the perfusion cycle desirable.
Transplantation of free tissue is one of the most complex procedures in modern transplantation medicine and reconstructive microsurgery. The transfer of free flaps is sometimes the only treatment option for the reconstruction of large and complex tissue defects due to traumatic injuries with exposed vital structures or resulting tumor resections. Techniques of replanting autologous free flaps have gained a vast increase in their level of sophistication, but it remains a complex surgical procedure. Free flaps suffer from ischemia between tissue harvesting and anastomosis of the adherent blood vessels. This poses a major threat to the tissue due to the ischemia reperfusion injury which can ultimately result in partial or total flap loss.
Materials & Methods
Muscular tissue shows a strong sensitivity to ischemic conditions compared to other types of tissue. Transgressing the critical ischemic time leads to a significant increase of apoptotic and necrotic damage. This study is part of a project to evaluate an extracorporeal miniaturized perfusion and oxygenation system that circumvents the problem of exposing vital muscular free flaps to prolonged times of ischemia due to intraoperative complications. For this purpose, the caudal segment of porcine rectus abdominis muscle harvested from mature German pigs was connected to the perfusion system. This muscle shows remarkable resemblance to its human analogue. The muscle´s blood supply is mainly realized through two major vessel pedicles, the superior epigastric artery at the cranial and inferior epigastric artery at the caudal end of the muscle with its respective veins. For perfusion experiments the muscle is cut between the lower intersections and only the caudal segment is used leaving the inferior epigastric artery and vein open for perfusion. The system itself consists of a clinically integrated infusion pump (Infusomat Space P, B. Braun Melsungen) drawing perfusion liquid from a reservoir and carrying it to the catheterized arteria epigastrica inferior of the banked muscle. Perfusate exiting from the venous branch of the epigastric pedicle is brought back to the reservoir closing the perfusion circle. For the online monitoring of oxygen flow-through cells containing chemical optical oxygen sensors (FTC-PSt3-L2.5, PreSens GmbH) are integrated in the arterial and venous flow of the perfusion system. The sensors are connected to a multi-channel oxygen meter (OXY-4 mini, PreSens GmbH). Monitoring oxygen is critical to the success of perfusing muscular tissue to prevent hypoxia. Sensors provided by PreSens are ideal for these purpose due to their fast response time and they are minimally invasive. Further advantages of these optical oxygen sensors are the independence from flow velocities and the absence of cross sensitivities with salt, pH values, or dissolved CO2 or SO2 in the medium. Perfusion flow is set to 600 mL/h to establish physiological intravascular flow rate and pressure. Initial experiments include the use of a crystalloid infusion solution (Jonosteril, Fresenius Kabi) and autologous blood. In both cases Heparin as anticoagulant agent is used (500 I.E./L).
Results
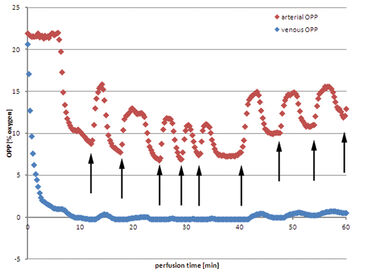
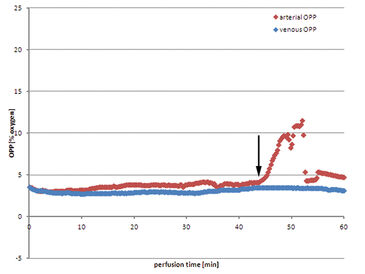
Using Jonosteril as perfusate a continuous flow of 600 mL/h can be successfully established in all experiments to ensure moderate intravascular pressures. The following data is a representative example for all experiments. Venous oxygen partial pressure (OPP) drops almost immediately after initializing the perfusion (Fig. 1). After nine minutes the value drops to 0 % OPP in the venous perfusate. On the arterial side OPP remains at a constant initial level, app. 21.0 %, and drops after six minutes as venous oxygen deprived perfusate is brought back into the reservoir due to the closed circular system conformation and mixes with the remaining fluid. Arterial OPP drops subsequently to a minimum of 8.8 % before fresh oxygen saturated perfusate has to be refilled into the reservoir to balance out perfusate loss through unavoidably injured minor vessels of the muscle while harvesting. Hence arterial OPP rises before oxygen concentration in the reservoir is diminished by continuous mixing with venous perfusate. This procedure repeats itself leaving arterial OPP fluctuating periodically, between 16.2 % and 7.4 %. Venous OPP on the other hand is mainly unaffected by these fluctuations and continues to remain at low values near 0 %. The tissue actively consumes oxygen. Using autologous blood as perfusate shows some major drawbacks. Despite the extensive use of heparin, coagulation in the perfusion system seemed to be unavoidable. The perfusion has to be initialized at lower rates of down to 400 mL/h to prevent high intravascular pressures before slowly raising the perfusion rate back to 600 mL/h. Arterial OPP remains at a low level from the beginning of the perfusion, between 3.0 % and 4.1 %. Venous OPP shows only slightly lower values, over the whole perfusion the value remains between 2.8 % and 3.5 %. Perfusate loss makes refilling the reservoir with fresh collected autologous blood necessary, but no increase in oxygen levels could be measured. After 42 minutes the huge amount of perfusate loss cannot be counterbalanced with harvested blood, so excluded perfusate is collected and reapplied to the perfusion system. The blood exposed to ambient air is saturated with oxygen, resulting in a rise in OPP after filling it into the reservoir. Venous OPP remains unaffected by the concurrent rise in arterial OPP.
Discussion
The perfusate solution plays an important role for supplying muscular tissue extra corporeally. Using a crystalloid solution a continuous flow can be established from the start, coagulation did not occur, and evening out perfusate leakage can be easily maintained. However, the formation of tissue oedemas fluid still represents a major drawback. Therefore in future applications the use of colloidal solutions is considered. In many applications for organ transplant preservations these solutions are used and it has been shown that the formation of oedemas can be reduced due to the lower colloid osmotic pressure. So far, a stable perfusion over the period of 1 h can be maintained. Longer perfusion times can be aspired due to initial results. The presented OPP values show that an additional complementary oxygenation unit is desirable, but it also shows that it is possible to attenuate hypoxia with perfusing the muscle extra corporeally with an oxygenated perfusate. For this purpose the use of a secondary oxygenation circuit to resupply diminished oxygen in the perfusate should be applied to ensure oxygen saturation and desaturation of carbon dioxide build-up. Another possibility of optimizing extra corporeal perfusion is the active regulation of tissue temperature to improve tissue preservation. Introducing immuno-histochemical evaluation of tissue samples during active perfusion to determine ischemic and apoptotic markers in the intent to improve perfusion results will provide additional insights in perfusion quality.
Application note adapted from Dragu A, Taeger CD, Buchholz R, Sommerfeld B, Hübner H, Birkholz T, Kleinmann JA, Münch F, Horch RE, Präbst K.., 2012 Jan 15., Online oxygen measurements in ex vivo perfused muscle tissue in a porcine model using dynamic quenching methods, Arch Orthop Trauma Surg. [Epub ahead of print]