Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Monitoring Skin Tissue Oxygenation in Mice
Comparing VisiSens with other Oxygen Measurement Methods
Julian Hofmann1, Robert M. Meier2, Alexander Mahnke1, Valentin Schatz1, Gregor Liebsch3, Otto Wolfbeis2, and Jonathan Jantsch1
1Mikrobiologisches Institut - Institute of Clinical Microbiology, Immunology and Hygiene, University Hospital Erlangen, Germany
2Institute of Analytical Chemistry, Chemo- and Biosensors, University of Regensburg, Germany
3PreSens GmbH, Regensburg, Germany
There is a great interest in getting more information on the role of tissue oxygenation in various diseases. In this study three different measurement techniques including the VisiSens oxygen imaging system have been used for quantifying skin oxygen tension in mice. Compared to measurements with Clark-type electrodes oxygen measurements with VisiSens are easy to perform and the system offers the great advantage of taking measurements non-invasively.
Well established pathological factors leading to severe tissue hypoxia are cancer and ischemia. Furthermore, infections and inflammation in a living organism are frequently associated with very low oxygen tensions in the afflicted tissues. Therefore, there is great interest in understanding the role of tissue oxygenation in various diseases. However, the role of tissue oxygen on course of various diseases can only be investigated if tissue oxygenation can be determined reliably. Various methods have been established for this purpose, but most of those do not provide exact values for tissue oxygenation. Thus, polarographic oxygen sensors are still considered to be the `gold standard´ for measuring oxygen tension. However, this method is invasive, the electrode itself consumes oxygen and thereby alters its oxygen microenvironment, and only single point measurements can be taken which makes it extremely difficult to map oxygen distribution in tissue in a reproducible manner. A recent development in oxygen sensing is the use of luminescent optical imaging using oxygen-sensitive dyes that quench luminescence in an oxygen-dependent manner. These dyes are immobilized in an oxygen permeable polymer matrix layer. The distribution of oxygen is determined by luminescence-based readout techniques, such as measurement of intensities, fluorescence lifetime (FLIM) or the signals at two wavelengths (ratiometry). Here, we wanted to compare three methods for quantifying skin oxygen tension in mice, namely the polarographic electrode technique and two systems that combine luminescent optical sensor foils with special imaging technologies - one being the VisiSens™ A1 imaging system.
Materials & Methods
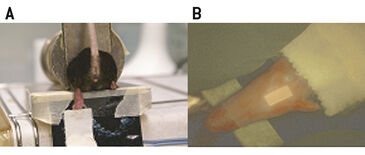
Female C57BL/ 6 mice were restrained and the hind leg was placed on a heating foil for approx. 5 min (40 °C) to promote diffusion of oxygen through the skin. The Clark-type electrodes (Ox-100, Unisense, Denmark) were two-point calibrated in either deoxygenated (using Na2S2O5) or air saturated water at 37 °C prior to use. Sharpened micro-electrodes (100 µm in diameter) were advanced into the heated skin of the footpad. A micromanipulator was used to measure in a standardized manner. The measurements were recorded twice a second over time and data was evaluated using the provided Sensor Trace PRO 3.1.3 software. Measurements using fluorescence ratiometric imaging (FRIM)-, or fluorescence lifetime imaging (FLIM)-based optical readout were performed as follows: Each mouse foot was fixed on a self-adhesive heating foil (Fig. 1A). A drop of water was deposited on the foot prior to covering it with a luminescent oxygen sensor foil. Thus, the foil is in close contact with skin and it is ensured that there are no air bubbles between sensor and foot that would result in erroneous measurements. The sensor was additionally fixed on top of the mouse foot using transparent adhesive tape (Fig. 1B). No excessive pressure was applied to the foot. Measurements were started after 5 minutes of equilibration time. FLIM was performed using a system comprising a time-gated CCD camera, pulsable 460 nm LEDs and optical filters. FLIM-sensor foils were precalibrated with different pO2-values generated by a gas-mixing device. Data were analyzed with the provided software (Image X TG1 v 4.0). The region of interest (ROI) of the measurement was defined as an area of 40 times 40 pixel in the middle of the adopted sensor. VisiSens™ sensor foils (SF-RPSu4, PreSens) were calibrated using Na2S2O5 prior to use. Signals were recorded in a darkened environment with the VisiSens™ detector unit DU01 prototype (FRIM-device) that was mounted on a tripod. Analysis of the data was performed with the provided VisiSens™ AnalytiCal 1 software.
Clark-type Electrode Measurements
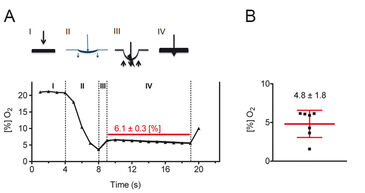
Figure 2 shows the results for measurements with a sharpened Clark-type microelectrode, which was advanced through murine skin using a micromanipulator. The pressure exerted by the tip of the microelectrode caused local ischemia of the skin tissue just before it penetrated the skin. After penetration, the local pressure and hence the pressure-induced local ischemia were immediately relieved resulting in an increase of the recorded oxygen saturation. The microelectrode was not further advanced in the tissue and the skin oxygen saturation was recorded over approximately 10 sec. Finally, the microelectrode was removed from the tissue. In summary, experiments using Clark-type electrodes revealed that the mean skin oxygen saturation in mice is 4.8 % ± 1.8 % (mean ± standard deviation, n = 7).
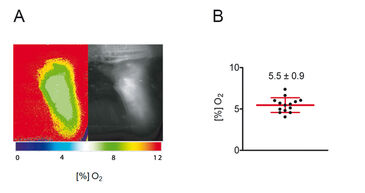
FLIM-based Measurements
Rapid lifetime imaging measurements with the FLIM-based imaging technology were performed and results are shown in Figure 3. In summary, these experiments revealed that mean skin oxygen saturation is 5.5 % ± 0.9 % (mean ± standard deviation, n = 14; Fig. 3B).
FRIM-based Measurements
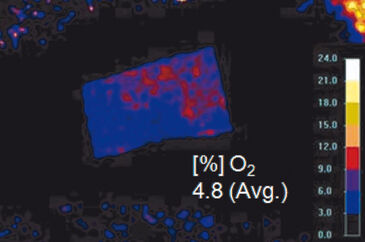
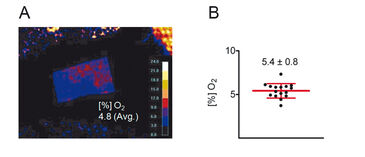
Results of ratiometric measurements with the VisiSens™ oxygen imaging system are depicted in Figure 4. In summary, these experiments revealed that the mean skin oxygen saturation is 5.4 % ± 0.8 % (mean ± standard deviation, n = 16; Fig. 4B).
Conclusion
We have validated the VisiSens oxygen imaging system against the Clark-type microelectrode and a FLIM-based optical oxygen imaging system for determining skin oxygen saturation in mice. Our data demonstrate that transcutaneous imaging of skin oxygen tension with VisiSens or the FLIM-based optical oxygen imaging system gives equal oxygen concentrations as invasive measurements with classical Clark-type microelectrodes. Furthermore, our values of skin oxygen saturation are in line with a report from Sheffield et al who observed that skin tissue displayed an oxygen saturation between 4 and 6 %. In contrast to Clark-type microelectrodes, oxygen measurements that are done with the VisiSens system are easy to perform. Furthermore, optical sensors allowed a non-invasive quantification of skin tissue oxygenation over a large area of skin. Therefore, the FRIM-based optical oxygen imaging system VisiSens is a perfect tool to analyze skin tissue oxygenation. In summary, this technology will advance our understanding of local regulation of tissue oxygenation and of its impact on the pathogenesis of cancer, infectious and cardiovascular diseases.
Application Note adapted from
J. Hofmann, R. J. Meier, A. Mahnke, V. Schatz, F. Brackmann, R. Trollmann, C. Bogdan, G. Liebsch, X. Wang, O. S. Wolfbeis, J. Jantsch, Ratiometric Luminescence 2D In-vivo Imaging and Monitoring of Mouse Skin Oxygenation; Methods Appl. Fluoresc., 2013, accepted