Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Optrode Dual Connects Single-Use Bioreactors to Conventional Controllers
Monitoring of CHO Cell Cultivation with Chemical Optical Sensors
Stephan Kaiser, Franziska Fietz, Nina Steiger, and Dieter Eibl
Institute for Biotechnology, Department of Life Sciences and Facility Management, Zurich University of Applied Sciences, Switzerland
The Optrode Dual™ prototype has been applied for measurements in suspension adapted CHO cell culture. The transmitter converts the reading of chemical optical sensors integrated in the cultivation bag into electrochemical signals, which are transferred to a conventional controller. CHO cell culture was successfully conducted in this set-up for 5 days. Correct functioning of the Optrode Dual™ throughout the whole cultivation period was verified even though operational errors occurred.
Most controllers are designed to work with electrochemical sensors for oxygen and pH monitoring. The Optrode Dual™ was developed to convert the reading of chemical optical sensors into an electrochemical signal (ECS) which can be transferred to the controller. This way the functionality of conventional controllers can be expanded to work with optical sensors for measurement of the important culture parameters oxygen and pH. The device also allows fail-safe calibration of optical sensors via 2D barcode control. In the following tests a prototype of the Optrode Dual™ was evaluated for its functionality in combination with the ez-control® by Applikon. Chemical optical sensors integrated in a cultivation bag were read out and their signal successfully converted and transferred to the controller. The combination of the systems of different manufacturers worked accurately.
Materials & Methods
The Optrode Dual™ prototype was connected to the ez-control® (Applikon, Netherlands) which was connected via Ethernet cable to a PC for data collection (Fig. 1). Data recording for dissolved oxygen and pH was realized with the software BioXpert (version 2.93.122b2) with a sampling rate of 1 min. Calibration of the optical sensors integrated in a 20 L cultivation bag was performed with the Optrode Dual™. Therefore, a specific barcode was generated with the software QR Code Generator in manual mode. The cultivation bag was placed on a BioWave 20 SPS platform (Wave Biotech, Switzerland). Suspension adapted CHO cells (CHO XM111-10, obtained from Fussenegger et al., ETH University, Zurich) were used for cultivation, which have a tetracycline regulated promotor for SEAP (secreted alkaline phosphatase) expression. However, only a growth experiment was performed without product formation. The cultivation bag was filled 3 h prior to inoculation with 1.5 L chemical defined medium (CHO Master HP1, Cell Culture Technologies) containing tetracycline (2.5 mg L-1) and Pluronic F68 (2 mg L-1). The medium was conditioned to 37 °C and aerated till it was saturated. The bioreactor was inoculated with 0.5 x 106 cells mL-1 with a starting volume of 2.4 L. Approx. 48 hours and 72 hours after inoculation 3 L, respectively 5 L of fresh culture medium were added. Angle and speed of movement of the cultivation bag were adapted to the filling volume of the bioreactor, to ensure optimal mixing. Samples of about 5 mL volume were taken four times a day. Cell count and determination of cell viability were performed automatically with NucleoCounter NC-100 (chemometec, Denmark). Furthermore, pH was measured off-line with a pH meter (Mettler Toledo, Switzerland).
Monitoring CHO Cell Culture
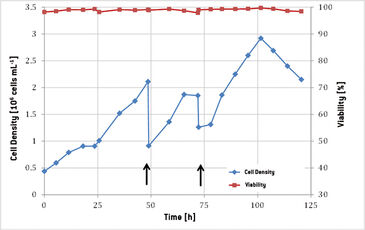
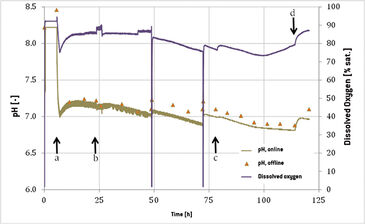
Functionality of the Optrode Dual™ during cultivation of suspension adapted CHO cells was tested in this set-up. The change of cell density and viability throughout the whole cultivation period is shown in Fig. 2. The initial density of 0.5 x 106 cells mL-1 increased to a value of 2.11 x 106 cells mL-1 within 48 hours, showing a growth rate of 0.03 h-1. 25 hours after inoculation cell growth was stagnating. The bioreactor movement rate of 15 rpm was raised to 19 rpm and gassing of 0.2 slpm (standard liter per minute) was set to 0.4 slpm (0.1 vvm to 0.2 vvm) which reestablished growth in the culture. After 48 hours fresh medium was added to the bioreactor and another period of exponential growth could be monitored with a doubling rate of 23 hours. 72 hours after inoculation another 5 L of medium were added to the bioreactor. Maximum cell density of 2.88 x 106 cells mL-1 was reached after 100 hours of cultivation. In the last 24 hours cell density was decreasing and reached 2.15 x 106 cells mL-1 with 98 % viability when the cultivation was terminated. Online recorded data for dissolved oxygen (DO) and pH are shown in Fig. 3. During the first 6 hours of cultivation a constant pH value of 8.22 was measured, because the calibration of the sensor was not conducted correctly due to an operational error. The barcode was read by the Optrode Dual™ but measurement was not restarted. Furthermore, the CO2 supply was not functioning correctly, so only after 10 hours the pH value could be adjusted to 7.2. Because of the increased streaming of CO2 into the bag the dissolved oxygen level decreased from 92 % to 77 % and was rising again after gassing was correctly adjusted to 0.2 slpm with 10 % CO2. A further increase in the DO level was recorded after 24 hours when gassing and movement of the bioreactor were changed. To determine the weight of the bioreactor during medium addition it was taken off the controlling system, this is why DO reading stopped at hours 48 and 72. At the same time the pH value rose with adding the slightly basic medium for 0.1 and 0.3 pH units, respectively. After 77 hours of cultivation another increase in DO could be investigated, caused by the second increase in gassing to 0.5 slpm. From hour 100 on DO increased from 75 % to 80 % due to decreasing cell growth. The last increase in DO was recorded after 110 hours of cultivation time, when CO2 supply was turned off. pH was measured offline to verify the online sensor reading. During the whole cultivation acceptable differences of below 0.2 pH units could be detected with offline determined values always being higher. This was probably caused by the time difference between sampling and measurement, during which pH regulating CO2 might have escaped the medium and caused pH to increase.
Conclusion
The test described here was designed to evaluate correct functioning of the Optrode Dual™ for monitoring CHO cell culture. The device was successfully connected to the optical sensors in the cultivation bag and accurate measurement data could be obtained. During tests no functional and technical errors occurred for the whole 5 day period. The Optrode Dual™ proved to be a reliable tool for measuring culture parameters with optical sensors and transferring data to a conventional controller. It is time saving as it can be easily connected to the electrochemical inputs of the controller and no further changes of controller settings are necessary. With the Optrode Dual™ it is possible to save costs as the purchase of new process analysis tools for reading optical sensors might not be necessary, and non-invasive monitoring of pH and oxygen can be conducted.