Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Studying Microbial Iron and Manganese Oxidation in Different Experimental Set-Ups
Non-invasive oxygen monitoring using a Fibox 3, OXY-4 mini and oxygen sensitive sensor foils
K. Laufer, M. Nordhoff, C. Scholze, M. Sezanne-Patzner, and A. Kappler
Center for Applied Geoscience (ZAG), Eberhard Karls University Tuebingen, Germany
At circumneutral pH three physiological types of microbial iron oxidation can occur, i. e. microaerophilic Fe(II) oxidation, anaerobic nitrate-dependent Fe(II) oxidation, and anaerobic phototrophic Fe(II) oxidation. We study all three types of microbial Fe(II) oxidation in different experimental set-ups to determine the contribution of microbial Fe(II) oxidation to the geochemical iron cycle. Other investigations focus on the role of Mn(II)-oxidizing bacteria in sand-based arsenic water filters, in order to ensure complete As removal from drinking water. Oxygen monitoring in the different set-ups is performed with chemical optical sensor foils by PreSens (SF-PSt3). They are glued to the inner surface of different experimental vessels and read out via polymer optical fiber from the outside with either a Fibox 3 or OXY-4 mini oxygen meter. This non-invasive operation principle and the small size of the sensors enables us to measure oxygen without disturbances or creating experimental artefacts. The examples presented here show the versatile application opportunities of these sensors in different geochemical applications.
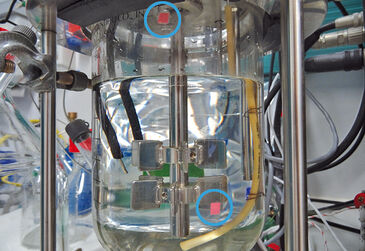
Fe(II)-oxidizing Bacteria Cultivated in a Chemostat
We are currently cultivating nitrate-dependent and phototrophic Fe(II)-oxidizing bacteria in a chemostat to analyse the physiology of these organisms. It is very critical to maintain strictly anoxic conditions in these cultures. Otherwise, Fe(II) will be oxidized abiotically by oxygen, and this will interfere with the determination of microbial Fe(II) oxidation rates. In this set-up it is advantageous to use the non-invasive sensors by PreSens for oxygen concentration measurements in the gas and liquid phase, because we can avoid the introduction of additional surfaces in the cultivation vessel that might create experimental artefacts.
Fe(II)-oxidizing Bacteria Cultivated in Artificial Sediment Columns
Many Fe(II)-oxidizing bacteria studied in our group were isolated from marine or freshwater sediments. Therefore, we use artificial sediment columns to cultivate mixed communities of phototrophic, nitrate-dependent and microaerophilic Fe(II)-oxidizing bacteria. The conditions in the columns resemble the bacteria´s natural habitat and provide gradients of light, oxygen and dissolved Fe(II) within the sediment. In these experiments it is essential to have a set-up that allows for oxygen gradients within the sediment to be measured at high spatial resolution and without disturbing the sediment or introducing additional pore space. The oxygen sensitive foils applied in our experiments withstand repeated autoclaving, freezing to - 80 °C and cleaning of the columns with 4 % HCl, which is necessary in our application.
Determination of Fe(II) Oxidation Rates in Microaerophilic Bacteria
At neutral pH and low oxygen concentrations (about 50 µM) microaerophilic Fe(II)-oxidizing bacteria are able to compete with the abiotic oxidation of Fe(II) by oxygen. It is critical to provide low but still sufficient oxygen concentrations to study the Fe(II) oxidation rates of these bacteria with minimal abiotic Fe(II) oxidation by oxygen. We intend to determine the Fe(II) oxidation rates of microaerophilic Fe(II) bacteria in an enrichment culture from a marine, littoral sediment using microcosms that contain quartz sand. The culture conditions aim to simulate the bacteria´s natural habitat where oxygen concentrations in the sediment are controlled by diffusion. Since it is necessary to maintain the correct, relatively low concentrations of oxygen in the microcosms the non-invasive oxygen monitoring method with PreSens sensors and oxygen meters is essential in these experiments.
Role of Mn(II)-oxidizing Bacteria in Sand-Based Arsenic Drinking Water Filters
Household sand filters are used in rural areas of Vietnam to remove As, Fe, and Mn from contaminated groundwater, and to ensure a safe drinking-water supply. The sand filters used in households in the Red River Delta region consist of two tanks. The upper tank is filled up with locally available sand. Freshly pumped groundwater percolates from the sand surface through the tank bottom. The filtered water enters through holes at the bottom of the tank or via an outlet at the front into the water storage basin. These filters immobilize poisonous arsenic via co-oxidation with Fe(II) and sorption onto or co-precipitation with the formed Fe(II) (oxyhydr)oxides. Unfortunately, so far no general indicator for the total removal of arsenic has been found. Manganese is a promising candidate to be used as an indicator of complete arsenic removal from the water. Mn(IV) oxides act as oxidants for Fe(II) and As(III). Mn(IV) is reduced to Mn(II) as long as Fe(II) and As(III) are remaining in the water. Mn(IV) oxides form a stable brown layer in the lower levels of the sand filter if As(III) and Fe(II) are completely removed in the upper sand layers. However, we found that in some cases Mn(IV) oxide reduction and Mn(II) mobilization was observed even when almost all As was removed from the water. Therefore, it was concluded that sand color is not a safe indicator for As removal. We will identify the geochemical conditions at which the Mn(IV) layer is formed by Mn(II)-oxidizing bacteria to ensure complete As removal from drinking-water. We use sand-filled glass columns that contain Mn(II)-oxidizing bacteria as a model of household sand filters in the laboratory. Oxygen-sensitive foils attached to the glass wall are used to measure oxygen depletion caused by microbial activity, over the column depth.