Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Oxygen Monitoring in Shake Flask Culture
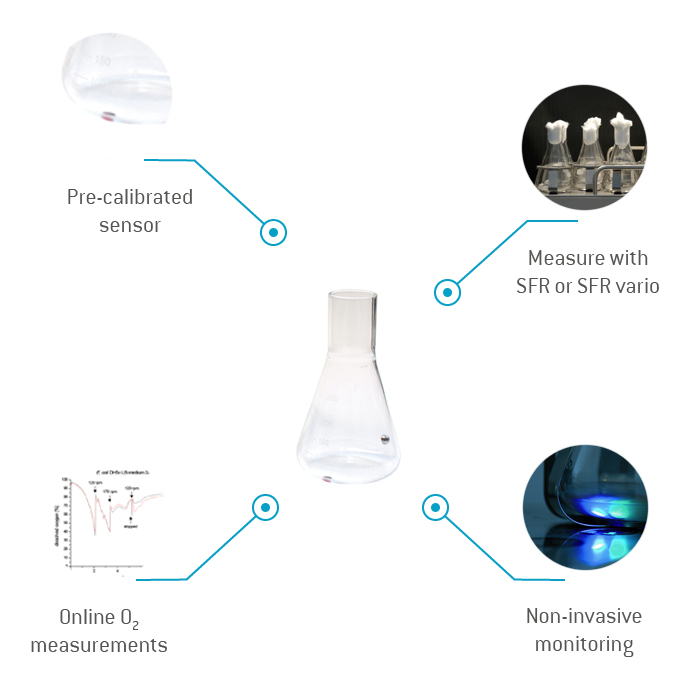
Glass Shake Flask with Integrated Sensor SFS-PSt3 (Glass)
PreSens offers glass flasks in volumes from 125 mL to 5,000 mL with integrated, autoclavable oxygen sensor at the flask bottom. The flasks are available with or without baffles and can be read out with the SFR Shake Flask Reader or the SFR vario (read-out with a coaster CFG and one of the Fibox or OXY series oxygen meters is also possible). The flasks are mounted on the reader in special clamps, so the sensor inside the flask can be aligned with the reader optics. The flask can additionally be equipped with a one-time autoclavable self-adhesive pH sensor, which can easily be replaced for new cultivations. Additionally PreSens offers a sensor integration service. Customer specified flasks can be equipped with autoclavable oxygen and one-time autoclavable pH sensors.
- Ready-to-use
- Pre-calibrated sensor
- Contactless measurement
- For microbes & cell culture
- Sensor integration service for customer specified flasks
Applications
Bioprocess Development: Oxygen Monitoring in Shake Flasks
O2 supply is one of the major issues in the cultivation of aerobic organisms. Shake flask cultures are widely applied in academic and industrial bioprocess development. As adequate methods for real monitoring of dissolved oxygen were missing, sufficient O2 supply is usually assumed. The non-invasive oxygen sensors in shake flasks now ensure oxygen supply and give new insights into metabolic activity.
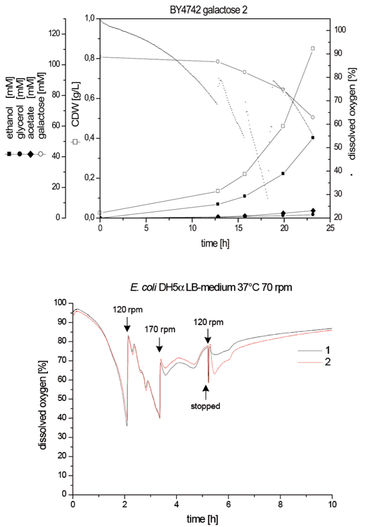
Yeast & E. coli: Ensure Enough Oxygen Supply
S. cerevisiae grows on different sugars as carbon source. While growth on glucose and fructose is mainly fermentative, growth on galactose is mainly respirative. This leads to low oxygen concentration in the shake flasks. The accurately measured oxygen indicates the need to increase rotation frequency to avoid oxygen limitation.
High oxygen demand is typical for E. coli in its exponential phase. In the cultivation shown on the left, rotation speed had to be changed twice in order to avoid oxygen limitation. In addition, changes in the metabolism can be detected by measuring DO.
Schneider et al., University of Saarland, Saarbrücken, GermanyBioprocess Biosyst Eng., 33(5), 541 - 547, 2009
Technical
| Specifications | Dissolved O2 |
|---|---|
| Measurement range | 0 - 100 % O2 |
| Limit of detection | 0.03 % oxygen |
| Resolution | ± 0.01 % O2 at 0.21 % O2 ± 0.1 % O2 at 20.9 % O2 |
| Accuracy | ± 0.05 % O2 at 0.2 % O2 ± 0.4 % O2 at 20.9 % O2 |
| Drift at 0 % oxygen | < 0.01 % O2 per day (sampling interval of 1 min.) |
| Measurement temperature range | from + 5 °C to + 50 °C |
| Response time (t90) | < 30 sec. |
| Properties | |
| Compatibility | Aqueous solutions, ethanol (10 % v/v), methanol (10 % v/v), pH 2 - 10 |
| Calibration | Pre-calibrated |
| Disposables are delivered irradiated | |