Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
How does temperature affect the oxygen measurement?
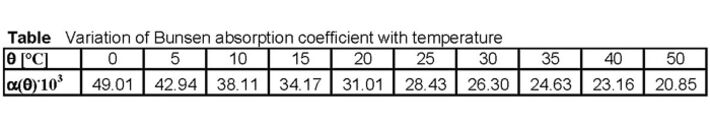
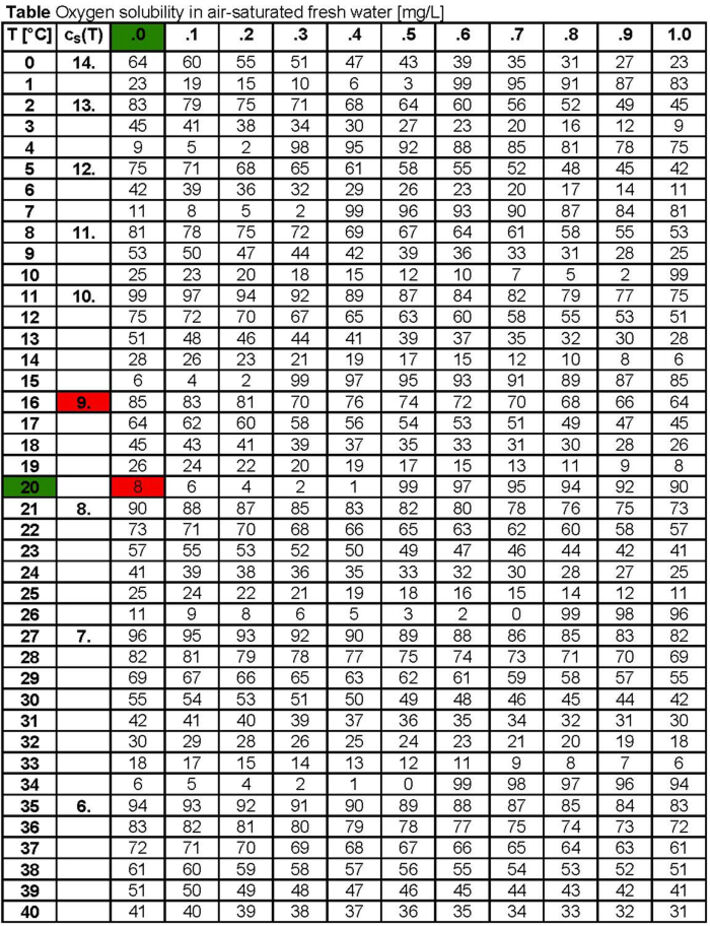
Like all physical parameters the fluorescence decay time and the Stern-Volmer quenching constant (= collision frequency) are dependent on temperature. The temperature behavior of these parameters is known and compensated. Beside this, the solubility of oxygen in water is temperature-dependent and can be described using the Bunsen absorption coefficient α(θ) and the oxygen partial pressure p(O2). With increasing temperature the solubility of oxygen in water decreases.
cS(p,θ) = [p(O2) - pW(T) / pN] α(θ)
cS(p,θ): temperature-dependent solubility of oxygen in water, given in (cm3(O2)/cm3)
p(O2): oxygen parital pressure
pN: standard pressure
α(θ): Bunsen absorption coefficient, given in (cm3 (O2)/cm3)