Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Non-invasive Method for Monitoring Real-Time Oxygen during Hematopoietic Stem/Progenitor Cell (HSPC) Culture
Timothy McKinnon1, Nelli Baal2, and Marek Zygmunt2
1Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada
2Universitätsklinikum Gießen und Marburg, Germany
The SDR SensorDish® Reader from PreSens has allowed for the quantification of in vitro oxygen concentrations during the culture of hematopoietic stem/progenitor cells (HSPC) isolated from human umbilical cord blood. The influence of simulated physiological oxygen concentrations on HSPC proliferation, cell cycle status, as well as expression of markers of stem cell phenotype, or differentiated cell types was examined concurrently with the monitoring of real-time oxygen concentrations in the cell culture media. This study has demonstrated the usefulness of this system for examining cell phenotype and function in conditions that effectively mimic the in vivo oxygen environment.
Oxygen concentrations change dramatically during human embryonic development. These alterations in oxygen concentration are most striking in the developing placenta. Trophoblast cells are one of the earliest cell types to form in the human placenta and, thus, must develop in a variety of oxygen concentrations. Similarly, hematopoietic and vascular progenitor cells can first be found within the early placenta in a low oxygen environment. As development proceeds, oxygen tensions rise and both trophoblast and progenitor cells adapt to the rising oxygen concentrations. Interactions between trophoblast and vascular cells are extremely complex and have not been adequately described. Alterations in oxygen concentration are known to have direct effects on the differentiation of trophoblast and vascular cells. Abnormal placental oxygen concentrations likewise affect growth and development of the fetus and could eventually lead to an increased risk of fetal and maternal morbidity and mortality. For this reason, it is extremely important that investigations into the effects of oxygen concentration on placental growth and vascular development continue. An in vitro culture system that effectively mimics these alterations in physiological oxygen concentrations during in vitro cell culture would prove irreplaceable in future studies.
HSPC Culture in Physiological Oxygen Concentrations
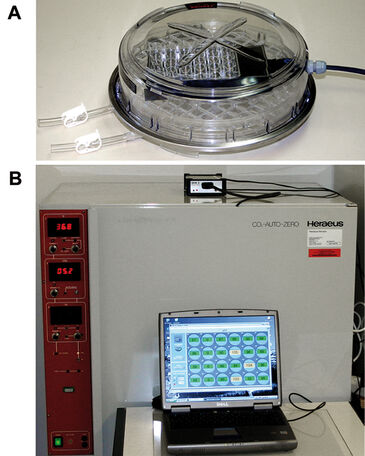
CD133+ HSPC cells isolated from human umbilical cord blood were cultivated for up to one week in 24-well OxoDishes® from PreSens. Every 2 -3 days, cells and culture media were removed, briefly centrifuged (1500 rpm; 5 min), and cells were carefully resuspended in media that had preincubated for 1 h in the appropriate oxygen concentration (1 %, 8 %, or 20 %). Low oxygen environments in this cell culture study were achieved using a hypoxia chamber (Billups-Rothenburg, USA) inserted into a traditional CO2 incubator (Fig. 1). This set-up allowed for specific physiological oxygen concentrations to be monitored and maintained in vitro. Real-time oxygen concentrations were monitored online with the SDR during the entire incubation period. Concurrently, HSPC cells were cultivated in either 24- or 6-well plates depending on assay, under identical conditions. When media was exchanged, an aliquot was removed and cell number determined using a Casy® Counter. Flow cytometric measurement of DNA content allowed for examination of HSPC cell cycle status following culture in physiological (1 %, or 8 %) or ambient (20 %) oxygen concentrations. Similarly, apoptosis levels were quantified using the FITX-Annexin V Apoptosis Kit (BD) in combination with flow cytometry. At the protein level, expression patterns of extracellular markers of stem/pro- genitor or differentiated cells were analyzed using multi-parametric flow cytometry (results not shown).
Results
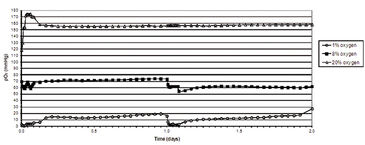
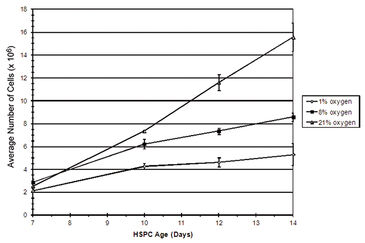
Figure 2 shows in vitro oxygen tensions measured with the SDR in combination with OxoDishes® during two-day HSPC culture in various oxygen environments. An average oxygen tension of 10.2 (±5.5) mmHg was encountered when HSPC were cultured in 1 % O2, and 65.0 (± 7.5) mmHg when kept in 8 % O2. In ambient oxygen (20 % O2) the average oxygen tension in HSPC culture media was 157.9 (± 9.2) mmHg. To obtain a relatively stable low oxygen environment, the atmosphere was replaced one time per hour for three hours at 24 hour intervals. In hyperoxic environments, HSPC displayed an increased rate of proliferation. HSPC growth curves decreased in an oxygen concentration-dependent manner, with the lowest cell proliferation seen in environments containing 1 % O2 (Fig. 3). Flow cytometric analysis of HSPC DNA content allowed for the analysis of cell cycle status. Results from this investigation clearly demonstrated that the number of cells either resting (e. g. G0) or in G1 increased significantly when oxygen concentration in the environment decreased. Oxygen concentration appeared to have no significant effect on HSPC apoptosis in this experiment (data not shown). Taken together, these results suggest that when HSPC are cultured in conditions that mimic the in vivo environment, cell proliferation decreases, most likely due to an increase in the number of resting cells (G0) and without any significant effects on cell death. This study demonstrates evidence that oxygen concentration has a definite effect on stem/progenitor cell phenotype and function and further substantiates the idea that oxygen plays a key role during placental development.
New Perspective for Non-Invasive Oxygen Monitoring
Physiological oxygen concentrations during distinct time-points in human placental development could be effectively mimicked with surprising precision using two different gas mixtures, containing either 1 % or 8 % O2 (5 % CO2). Measurements made in this study with the SDR paired with OxoDishes® from PreSens provide additional proof that traditional cell culture in atmospheric oxygen (20 % O2) creates a clearly hyperoxic environment with oxygen tensions well above normal pO2 found in human tissues. The use of low oxygen gas mixtures is not novel in placental research. One important difference, however, is that previous investigations did not include non-invasive, real-time oxygen monitoring. As clearly demonstrated in this investigation, oxygen concentrations in low oxygen cell culture systems are in a constant flux and are difficult to maintain at physiological levels. Accordingly, standardization of experimental parameters (e. g. cell number, media composition, gas replacement intervals, etc.) and extensive testing was required to ensure that actual physiological oxygen concentrations were reached and subsequently maintained. Using standardized protocols, variability between experiments was reduced. Furthermore, the SDR oxygen monitoring system provided reliable data concerning in vitro oxygen levels during the entire experimental period. Reproducible results from this study have demonstrated a clear connection between oxygen concentration, cell proliferation, and cell cycle status. The significance of these results on stem/progenitor cell culture and function will be examined in detail in the future. The SDR has opened up the possibility of further in vitro studies in which defined, physiologically relevant oxygen concentrations, and stable cell culture conditions play an important role. Furthermore, media changes, or alterations in oxygen concentrations can be monitored and altered according to experiments. Using this system, research objectives will be reached in a timely manner, thereby reducing research cost.