Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Christoph Neubrand, Johannes Wirth, Dietmar Lang, Marvin Kadisch, Konstantin Bagnjuk
Rentschler Biopharma SE, Laupheim, Germany
A fed-batch-process with Horizon’s glutamine synthetase knockout CHO cell line was carried out in an autoclavable 10L benchtop bioreactor. For online culture monitoring PreSens’ novel pCO2 measurement system - with the sensing element installed within a bypass - was applied. The online measurements were in good agreement with gold standard Bioprofiler’s offline pCO2 measurements.
Monitoring cell culture parameters, like dissolved oxygen concentration, pH and pCO2 is essential to ensure product quality and yield. Small changes in the culture environment can have an influence on cell metabolism, viability and even on glycosylation patterns of mABs. In mammalian cell culture typically low gassing rates will be applied to prevent the media from foaming and the cells from the shear stress of bursting bubbles. A variety of gassing strategies have been developed to allow for proper oxygen supply, while keeping stress and foam formation low – especially in production scale bioreactors (> 250L) - these strategies are prone to the accumulation of dissolved CO2, which will usually be countered by stripping the media with nitrogen or air. For the stripping the bioreactor-controller will need online pCO2-information, generating a demand for reliable pCO2 measurement technology. Therefore, a novel measurement system was evaluated, that allows determination of pCO2 levels in the culture medium in real time, in a 10 L scale-down model process.
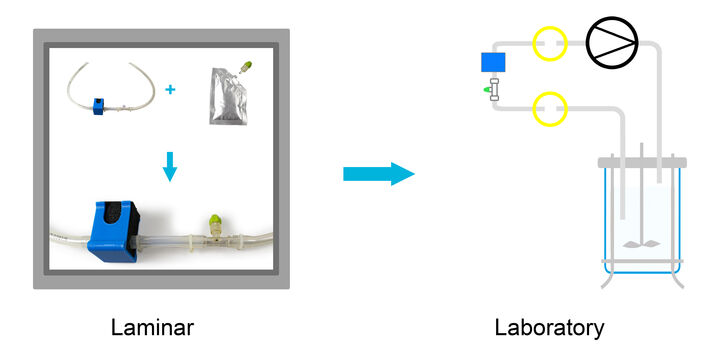
The system consists of three components: (i) a ¼” C-Flex hose with an integrated flow-through cell and temperature sensor, which come irradiated and can be installed in a bypass, (ii) an optical pCO2 sensor, immobilized on the distal end of a sensor stick, which is packed separately in a squeeze bag with physiological saline solution, and which can be inserted in the flow-through cell via a Luer connector under laminar flow, (iii) an optical pCO2 meter for pCO2 and temperature measurement, which is controlled via PC (4-20 mA output module available, but not used in this study). The separate components allow for safe and easy installation under laminar flow. When the bioreactor setup is ready the optical pCO2 sensor and temperature sensor can then be connected to the CO2 meter. This system for pCO2 monitoring was tested in a CHO fed-batch process and referenced against offline measurements.
Materials & Methods
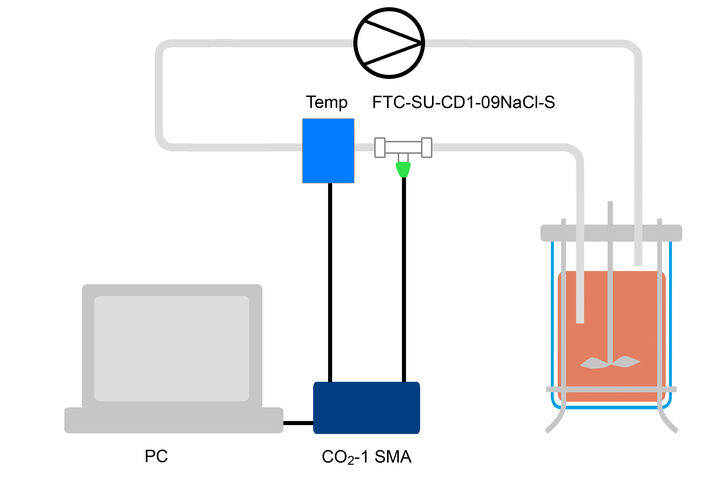
CHO GS knockout cells (HorizonTM) were grown in standardized mammalian cell culture medium in a 10L stirred-tank glass bioreactor. The fed-batch process was carried out with gassing on demand and a temperature setpoint of 35.5 °C before and 32 °C after temperature shift at day 6 of cultivation. Temperature and pCO2 were monitored online with a measurement set-up developed by PreSens. A ¼” C-Flex-assembly with integrated flow-through cell and temperature sensor was installed in a bypass. The CO2 sensor stick, and the temperature sensor inserted in the C-Flex-assembly were connected to the CO2-1 SMA fiber optic measurement device for online monitoring. Culture medium was pumped through the bypass at 60 mL/min with a peristaltic pump. The cell count (Cedex HiRes, Roche CustomBiotech), pH and pCO2 (Bioprofiler pHOx, Nova) were determined via daily offline measurements.
Results
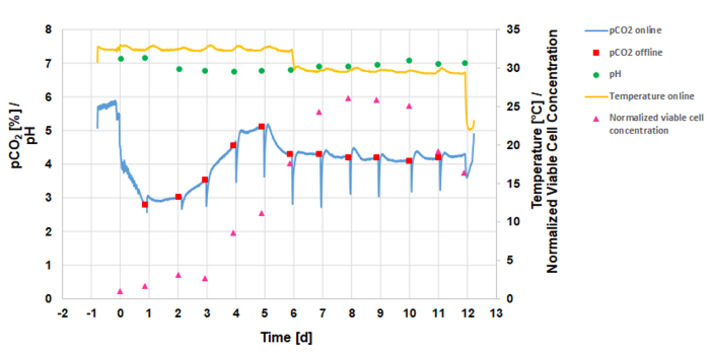
Figure 3 shows the online and offline determined culture parameters in the CHO fed-batch process. The maximum normalized viable cell concentration of 2.61 was reached at day eight. The temperature measured online in the bypass with the PreSens measurement system was approximately 3.5 °C below the temperature setpoints of 35.5 °C before and 32 ° C after the temperature shift. However, the online measurements clearly showed the temperature shift at day 6 which was performed to prolong the cells’ viability and productivity. The process was performed at pH 7. The pH value slightly droped during the first 5 days of cultivation, due to metabolic activity of the cells.
During the first five days of cultivation the pCO2 level in the culture medium slowly increased from 3 % to 5 %. The dips in the online pCO2 measurements were caused by the daily substrate additions diluting the culture medium and decreasing the pCO2 level. After the maximum viable cell concentration was reached the pCO2 concentration remained at 4.2 ± 0.1 %. The graph shows that online measured pCO2 values were in good agreement with the daily offline pCO2 measurements.
Conclusion
The evaluation of the new PreSens pCO2 measurement setup for bioreactor bypasses showed that this system delivers accurate real-time data for culture control. The C-Flex-connectivity of this customized loop with optical measurement technology allows for safe installation of the sensor to autoclaved benchtop or single use bioreactors, as long as they are outfitted with the proper C-Flex-tubing counterpart.
Acknowledgement
The project leading to this application has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement n°777397. This Joint Undertaking receives the support from the European Union’s Horizon 2020 research and innovation program and EFPIA.
Disclaimer
The communication reflects the author's view and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.