Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Marques M.P.C., Cabral J.M.S., Fernandes P.
Institute for Biotechnology and Bioengineering, Centre for Biological and Chemical Engineering, Instituto Superior Técnico, Lisboa, Portugal
A key factor when optimising and developing bioprocesses is the monitoring of the fermentation, in order to quantify in real-time basis parameters like oxygen, pH and carbon dioxide, among others. This monitoring by using SDR allows the time course of the fermentation to be followed and process parameters to be adjusted to desired levels. Online monitoring in stirred vessels, namely fermenters, is a widespread approach but it has seldom been applied in shaken systems due to technical limitations.
Non-invasive Oxygen Detection
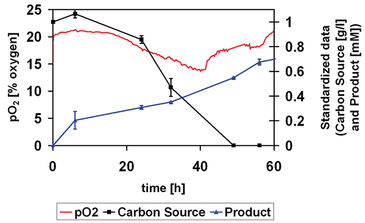
The SDR SensorDish® Reader (Figure 1) enables non-invasive online monitoring of oxygen and pH in 24-well microtiter plate fermentations. In the present work this device was challenged to monitor the time course of a multi-step biotransformation in both aqueous (Figure 2) and organic-aqueous two-liquid phase systems (Figure 3). Sitosterol side-chain cleavage to androstenedione (AD) performed by Mycobacterium sp. NRRL B-3805 was selected as a model system (Marques et al., 2007).
Materials & Methods
Mycobacterium sp. NRRL B-3805 cells were grown using 24-well multidishes with integrated oxygen sensors (OxoDishes®) and incubated for a period of approximately 60 hours under 200 rpm orbital shaking at 30°C. The synthetic aqueous culture medium used was previously defined (Marques, et al., 2007). In order to prevent evaporation of the medium, AeraSeal sealing tape was used. The oxygen concentration in the culture medium was monitored online throughout the whole culture period using the SensorDish® Reader software. Oxygen consumption was compared with the carbon source depletion and product formation measured off-line by HPLC (Marques et al, 2007), using a sacrificial well technique. In terms of solvent selection, the cells were grown in the presence of a second phase composed of several organic solvents. Growth was monitored by oxygen consumption.
Precise Oxygen Consumption Profiles
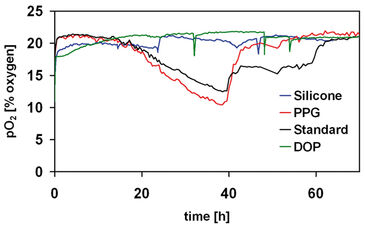
The oxygen consumption profile (Figure 2) clearly gives an idea of the different growth phases of the fermentation. A lag period of about 15 hours is observed, since oxygen and substrate levels are roughly unaltered. After this period, significant decay of oxygen and carbon source levels was observed, alongside with product formation. Finally, cells enter the stationary phase, as indicated by the increase in the pO2 concomitant to carbon source exhaustion. The effect of the nature of the solvent used as substrate carrier on mycobacterial growth was also assessed with the SensorDish® Reader technology for this particular biotransformation (Figure 3). Solvents such as silicone, dioctyl phthalate (DOP) and propylene glyco (PPG) were tested and growth was compared with growth in conventional medium (standard). Previous studies showed that DOP could interact chemically with microtiter plates (Marques, et al., 2007). With the OxoDish®, a similar phenomenon happened, however it did not tamper with the sensor for oxygen monitoring. This was confirmed by abruptly stopping the agitation and assessing the response. The decay and subsequent recovery of the oxygen levels to the initial values after the stopping indicates the level of response. This technique was also used to confirm the response of the sensor when in the presence of silicone. In both solvents, cells did not grow. Propylene glycol was confirmed as a suitable solvent (Stefanov et al., 2006) as indicated by the identical growth profile obtained when cells were grown in the absence of organic solvent.
Monitoring the Time Course of Fermentation
Both results show the robustness and flexibility of the SensorDish® Reader technology as a suitable tool for monitoring the time course of fermentation, even in non-conventional media. Data obtained closely matched those gathered in conventional devices, namely stirred fermenters and large shake flasks (data not shown), thus contributing to make microtiter plates the basis for representative screening systems, assuring reproducibility throughout scales. This capacity indicates the suitability of this platform to conduct biocatalytical studies in complex biotransformation systems, e. g., to develop and improve the process itself. Since the SensorDish® Reader allows a high number of parallel runs it is possible to obtain the desired result in a faster way, reducing not only associated costs but also human resources.
References:
Marques, M. P. C., de Carvalho, C. C. C. R., Claudino, M. J. C., Cabral, J. M. S., Fernandes, P. (2007) On the feasibility of the microscale approach for a multi-step biotransformation: sitosterol side-chain cleavage. J. Chem. Technol. Biotechnol., 82, 856 - 863.