Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Online pH Measurements in iTube pH Bioreactors
Evaluating the ITR iTube96Reader for Monitoring Insect Cell Culture Growth for Biopesticide Production
Ina Dittler1, Gernot T. John2, and Regine Eibl1
1Zurich University of Applied Sciences, School of Life Sciences and Faciltiy Management, Institute of Biotechnnology, Wädenswil, Switzerland
2PreSens Precision Sensing GmbH, Regensburg, Germany
In our experiments we tested TubeSpin50 bioreactors with integrated pH sensors (referred to as iTube pH bioreactors). We evaluated their suitability for growing Lymantria dispar Ld652Y-RH suspension cells. Furthermore, the accuracy of the ITR iTube96Reader, which allows parallel online pH monitoring in up to 96 iTubes pH, was tested. The online measured pH values gathered with the ITR were compared to offline pH measurements with a benchtop pH meter (Mettler Toledo). Finally, we determined cell growth and viability daily throughout the culture period of 11 days. Analysis of the samples revealed an expected growth peak cell density of 3.0 x 106 cells mL-1 and viabilities over 92 % of the insect cells inside the cell culture tubes. Online and offline measured pH values showed very good accordance (deviation below ± 1 %).
Insect cells and the baculovirus expression vector system (BEVS) are increasingly being used for the production of biopharmaceuticals and biopesticides. Compared to mammalian cell culture, insect cells are easier and cheaper to cultivate. Furthermore, their products need less production time and they can be handled within an environment with low biosafety level (level 1) [1]. The insect cells grow at a temperature of approx. 27 °C and have higher tolerance to osmolarity or by-product concentrations. They require no additional CO2 and tolerate pH values between pH 6.0 and 6.8. For screening studies and process development work based on insect cells and the BEVS, TubeSpin50 (Techno Plastic Products AG) bioreactors are recommended by various authors [e. g. 2]. PreSens have recently equipped these small-scale bioreactors with non-invasive pH sensors (iTubes pH). In our experiments we tested iTubes pH for the first time for their suitability to monitor pH in a L. dispar model cell line. The corresponding ITR iTube96Reader, which was placed directly inside the shaker incubator, allowed parallel pH measurements in up to 96 iTubes pH. Read-out of the sensors inside the tubes was controlled and recorded with the ITR software. In order to evaluate the ITR monitoring system, we compared online values measured with the device to offline measurements performed with a benchtop pH meter. To study cell growth, we took daily samples to determine total and viable cell density (TCD, VCD), viability, and substrate and metabolite concentrations.
Materials & Methods
We worked with the Ld652Y-RH cell line which was provided by Prof Dr James Slavicek (U. S. Forest Service, USA). The cells for the inoculum and the batch cultivation were propagated in modified ExCell420 (Sigma-Aldrich) insect cell medium with 5 % Serum Plus Media Supplement (Sigma-Aldrich) and 50 µg L-1 gentamicin solution (SigmaAldrich). The inoculum was produced in one TubeSpin600 (Techno Plastic Products AG) bioreactor at maximal working volume of 200 mL and a starting VCD of 0.5 x 106 cell mL-1. It was incubated in a shaker (Infors HT Multitron) at 180 rpm and 25 mm shaking diameter. On day 6 the culture reached a VCD of 1.4 x 106 cells mL-1 (exponential phase) and were seeded into iTubes pH with an initial VCD of 0.2 x 106 cells mL-1. Before seeding, the pH sensors were equilibrated with 5 mL medium for three hours. The inoculated iTubes pH were placed in the rack of the ITR which was connected via Bluetooth with the computer and the respective reader software. The cells were cultivated in the iTubes with a working volume of 20 mL, at 27 °C and 250 rpm. Over an experimental period of 11 days two iTubes pH (double determination) were harvested daily for offline analysis. TCD, VCD as well as viability were determined using NucleoCounter 100 (Sysmex). The substrate and metabolite concentrations were quantified with the BioProfile 100 Plus (Labor-Systeme Flückinger AG) automatic analyzer. Besides online pH measurements, the offline pH value was measured with a pH meter (Mettler Toledo), that was calibrated using pH 4.01 and pH 7.0 buffers before each measurement.
Insect Cell Culture in iTubes pH
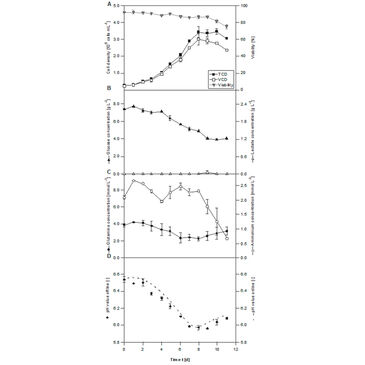
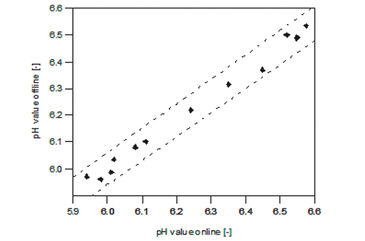
Daily sampling and offline data analysis of Ld652Y-RH cells inside the iTubes pH showed expected growth and viability (Fig. 2A). VCD increased to about 3.0 x 106 cells mL-1 within 8 days. Then the cultures turned into stationary phase, while VCD decreased slightly (11 %). Viability of over 92 % could be maintained for 5 days and stayed above 85 % until day 9 of cultivation. The maximal growth rate and doubling time for the exponential phase were calculated to be 0.021 h-1 and 33.2 h. The obtained cell number is in good agreement with data reported for the LdFB cell line in 250 mL shake flasks [3]. A lower maximal VCD of LdElta (1.5 x 106 cell mL-1) has been described by Betenbaugh et al. [4], whereby the maximal growth rate of about 0.019 - 0.024 h-1 and the maximal doubling time of 28 - 35 h are well comparable with our results. Figures 2 B, C show the metabolite profile of Ld 652Y-RH cells, which is similar to those of the Spodoptera frugiperda cell line Sf-9. The glucose concentration decreased continuously from the initial value of 7.3 g L-1 to 4.0 g L-1 within 11 days. The concentrations of glutamine decreased from 3.8 mmol L-1 to 2.2 mmol L-1 during the exponential phase and increased to 3.2 mmol L-1 in the stationary and the death phase. The maximal ammonium concentration of 2.6 mmol L-1 was measured on day 1, which decreased to 0.7 mmol L-1 until day 11. The pH values in the L. dispar cell culture constantly decreased from pH 6.5 to 5.9 until day 9 (Fig. 2 C). When growth stopped and the number of viable cells decreased (Fig. 2 A), pH increased again slightly (3 %) (Fig. 2 D). The comparison of pH values (Fig. 3) obtained online with the ITR and offline values from daily sampling showed good accordance with a deviation below ± 1 %. This demonstrates that accurate pH monitoring can be performed with the ITR during L. disparbased processes.
Conclusion
The functioning of the ITR iTube96Reader in combination with iTubes pH with integrated non-invasive sensors was successfully evaluated for the Lymantria dispar cell line Ld 652Y-RH in a pH range between 5.9 and 6.5. During the cultivation, the ITR system revealed stable pH measurements (± 1 %) without functional and technical problems.
Acknowledgement
The authors would like to thank Prof Dr James Slavicek (U. S. Forest Service, USA) for providing the L. dispar cell line and Nancy Hayes-Plazolles (U. S. Forest Service, USA) for technical support.
References
[1] R. Eibl, et al. (2014), in Disposable Bioreactors II, Springer, 99 - 125
[2] M. J. De Jesus, et al. (2004), Biochemical Engineering Journal 17, 217 - 223
[3] J. Vaughn, and F. Fan (1997), In Vitro Cellular & Developmental Biology-Animal. 33, 479 - 482
[4] M. J. Betenbaugh, et al. (1991), Biotechnology Progress. 7, 462 - 467