Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
How does salinity affect the oxygen measurement?
The solubility of oxygen in water is dependent on salinity, while the partial pressure and the % saturation of oxygen is not affected by changes in salinity. This means that in absolute concentration a seawater sample will contain less oxygen than a freshwater sample at the same temperature although the partial pressure is the same.
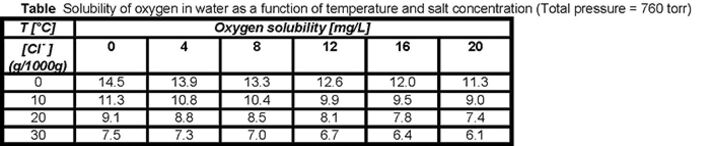
The table below lists values of the concentration of dissolved oxygen at several temperatures in solutions with various chloride concentrations. Increasing the salt concentration leads to a decrease in oxygen solubility. This behavior is characteristic for the solubility of many non-electrolytes - this phenomenon is known as salting-out effect.
Instead of chlorinity [Cl-] - the amount of chloride in parts per thousand - which was used as a measure of the amount of salt in water, the term salinity is often used. If salinity is preferred as a measure of salt concentration, then the conversion from g/L can be readily made using equation 1.
S = 0.1805 [Cl-] + 0.003 where S is the salinity in [%] or [g/1000g] (1)
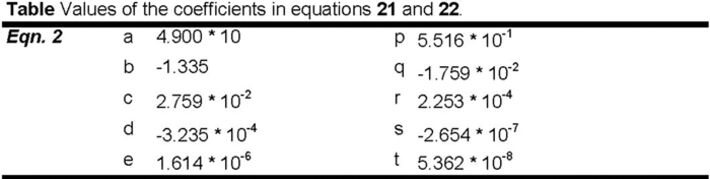
The dependence of oxygen solubility on salt concentration can be obtained from equation 2.
103 ⋅ α = a + b ⋅ θ + c ⋅ θ2 + d ⋅ θ3 + e ⋅ θ4 - [Cl-] ⋅ (p + q ⋅ θ + r ⋅ θ2 + s ⋅ θ3 + t ⋅ θ4) (2)
where θ is the temperature in °C, a - e are the coefficients used in equation 16 and p - t are new constants given in the table below. The values of these new constants are obtained by fitting the polynomial to experimental data in the ranges 0 ≤ θ ≤ 30°C and 0 ≤ [Cl-] ≤ 0.2 %. To obtain oxygen solubility from the Bunsen absorption coefficient, the same procedure as described previously is used.