Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Hypoxia in Static and Dynamic 3D Culture Systems
Oxygen Gradient Measurements in 3D Culture Systems Designed for Bone Tissue Engineering
Elias Volkmer1, Inga Drosse1, Sven Otto2, Achim Stangelmayer3, Michael Stengele1, Bobby Cherian Kallukalam1, Wolf Mutschler1, and Matthias Schieker1
1Experimental Surgery and Regenerative Medicine, Department of Surgery, LMU Munich, Germany; 2Department of Oral and Maxillofacial Surgery, LMU Munich, Germany
3PreSens Precision Sensing GmbH, Regensburg, Germany
Gradients in tissue quality from the periphery towards the center are a limiting factor in tissue engineering of sizeable cell-scaffolds. Uneven oxygen supply might be the cause for irregular cellular growth on scaffolds. In this study continuous measurements of oxygen concentration in the center of scaffolds designed for bone tissue engineering and the surrounding medium were performed. In static 3D culture systems the oxygen concentration in the center dropped to 0 % after 5 days and resulted in cell death. Perfusion bioreactors can prevent cell death but still do not entirely eliminate 3D culture-associated oxygen gradients.
Engineering bone tissue in vitro in clinically relevant extents has not been successful so far. Inhomogeneous cellular proliferation and differentiation from outer areas of the scaffolds towards the center occur with increasing size of the cell-seeded scaffolds. The bigger the cell-seeded scaffolds are the more difficult it is to supply all cells equally with nutrients and oxygen. This study investigated whether 3D culture systems commonly used for bone tissue engineering are generally affected by oxygen gradients, and whether these gradients have an impact on cell survival within the construct. Chemical optical oxygen microsensors (PreSens GmbH, Regensburg, Germany) were applied to measure oxygen concentrations in the center of dynamic and static 3D culture systems.
O2 Concentration Measurements
A subclone of the murine preosteoblast cell line MC3T3-E1 (DSZM, Braunschweig, Germany) was expanded in minimum essential medium alpha with L-glutamine (Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum (FBS; Sigma, Munich, Germany) and 40 IU/mL penicillin/streptomycin. Cells were kept in a humidified atmosphere of 95 % air with 5 % CO2 at 37 °C and supplied with fresh medium three times a week. Cylindrical, sterilized, bovine demineralized bone matrix (DBM; Tutogen Medical, Neunkirchen, Germany) scaffolds of 9 mm in diameter and 5 mm in height served as matrix in all experiments. 666 µL of cell suspension (approx. 7.5 x 104 cells per mL) were transferred onto the scaffolds. Needle-type oxygen microsensors with fixed sensor tip (PreSens) were used for oxygen measurements in the 3D cultures. For static culture, the seeded scaffolds were kept in a standard cell culture incubator in 24-well-plates. To continuously monitor oxygen partial pressure in statically cultured scaffolds, the needle-type sensor was passed through a self-made hole in the lid of the culture plate and inserted exactly in the center of the freshly seeded scaffold at a depth of 2.5 mm from the top in a rectangular fashion. Oxygen was measured every hour over a period of 7 days. To measure oxygen in the medium another oxygen probe was placed next to the scaffold. For dynamic 3D culture two set-ups of perfusion bioreactor culture were used: In the standard perfusion set-up the medium could flow around the scaffold, as the flow chamber was bigger in diameter than the used scaffold. For a forced perfusion set-up carrier cassettes of polycarbonate were constructed that carried the scaffolds in a press-fit fashion and exactly fitted into the flow chamber. This way the culture medium was forced through the scaffold. The bioreactors were connected to both fresh medium containing 25 mM HEPES buffer (PAA) and to waste reservoirs by gas-permeable silicone tubes. In the standard perfusion set-up the oxygen microsensor was placed halfway from the top in a depth of 4.5 mm in the geometrical center of the construct. For oxygen measurement in the forced perfusion set-up holes were drilled in the side of the polycarbonate cassette to insert the oxygen probe and place it in the center of the scaffold. To measure oxygen in the medium of the perfusion bioreactor cultures additional oxygen probes were inserted, one in the afferent and one in the efferent medium reservoir. To assess survival of cells fluorescence microscopy based on incubation of cells with fluorecein-diacetate (FDA) and propidium iodide (PI) (Fluka/Sigma, Munich, Germany) was performed.
Hypoxia in 3D Culture
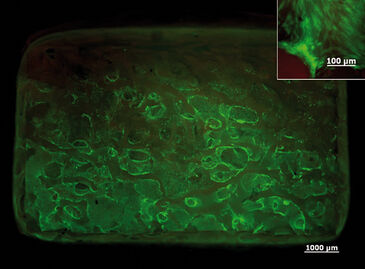
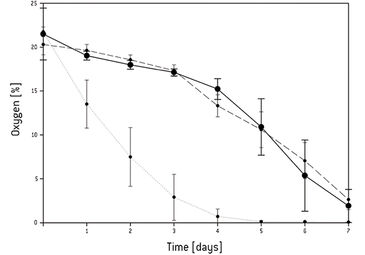
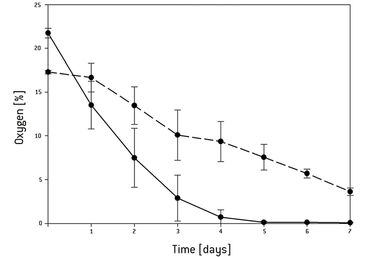
Continuous oxygen measurement in the static 3D culture revealed that an oxygen gradient had formed from the surface of the 3D construct towards the center. After 5 days the oxygen concentration dropped to 0 % in the center of the scaffold and 4 % in the surrounding medium [Fig.1]. The live/dead assay showed that after culturing 3 days all cells were still viable, and after 5 days most of the cells still were even though the oxygen concentration dropped as low as 1 %. After 7 days the core and the base of the scaffold were filled with dead cells. In the center of the standard perfusion culture less cells had died after 7 days compared to static culture conditions. Repeating the experiment under the same conditions in the forced perfusion, the viability-stain performed after 7 days showed that even more cells had grown in the center and the periphery of the scaffold in this set-up. Only in the top region of the scaffold fewer cells seemed to have grown. Higher magnification of the respective area, however, revealed decreased but satisfactory cell proliferation [Fig. 2]. In the forced perfusion culture oxygen concentrations of 20 % at the inlet and about 12.5 % at the outlet could be measured. But in both dynamic culture set-ups the oxygen level had decreased in identical manner to 4 % in the center [Fig.3].
Conclusion
Cells with high proliferative potential create an oxygen gradient within scaffolds when used for 3D culture. Data obtained in this study, using a fast growing mouse cell line cannot be directly transferred to human cells. These cells were chosen as they are commonly used as a model cell line to assess bone cell metabolism. Furthermore with these fast growing cells the different culture set-ups could be challenged so even limitations in dynamic 3D culture could be detected. Static 3D culture showed to be an inapplicable method to generate tissue. The flow perfusion improves the oxygen supply to cells and therefore enhances cell proliferation. But still the oxygen concentration in the center of dynamic culture is significantly lower than at the inlet and outlet of the bioreactor, so oxygen limitation is not completely eradicated and a homogeneous oxygen supply is not guaranteed. Engineering sizable tissues will require careful monitoring of the oxygen levels within the cell-scaffold constructs. Measuring the central oxygen concentrations seems to be minimum requirement for all scaffold-based tissue engineering activities.
Application note adapted from
Volkmer et al., 2008, Hypoxia in Static and Dynamic 3D Culture Systems for Tissue Engineering of Bone, Tissue Eng: Part A 14,
No. 8