Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Impact of dCO2 on Process Parameters
Effect of High dCO2 on Viable Cell Density and Product Titer in CHO Cell Culture
Jennifer Couture, Jennifer Lindsay, and Rick Baggio
EMD Millipore Corporation, Bedford, MA, USA
Real-time monitoring of bioprocess parameters is important to ensure reproducible cell culture and optimize product quality. Fast detection of unfavorable conditions in cell culture requires reliable sensor systems. CHO cells - producing and secreting an antibody - were cultivated in bioreactors and viable cell density and product titer were monitored. Then content of dissolved carbon dioxide in the culture medium was monitored with an optosensoric CO2 measurement system.
Choosing reliable sensors for in-line measurement of process parameters can help to detect and control unfavorable culture conditions. Small changes in e. g. oxygen supply, pH, or CO2 in the culture medium can lead to unexpected changes in cell metabolism and viability. This can minimize the production yield. In the following test viable cell density, production titer and dCO2 were monitored in CHO cell culture. The fiber optic CO2 transmitter pCO2 mini by PreSens Precision Sensing GmbH, Regensburg, Germany - applying chemical optical sensors - was used to monitor dCO2 during bioreactor runs. Using this system it was possible to show that high dCO2 content in the culture medium can cause growth lag.
CHO Cultivation
The CHO cells used in these experiments produced and secreted an in-house humanized variant of the anti-phosphotyrosine monoclonal antibody 4G10® clone (EMD Millipore). Cells were cultivated in Applikon® ez-Control (Applikon Biotechnology Ltd., Dover, NJ) bioreactors of 3 L, working volume 2 L. The medium used for the bioreactors was Gibco® OptiCHO™ medium (Life Technologies Inc., Carlsbad, CA).
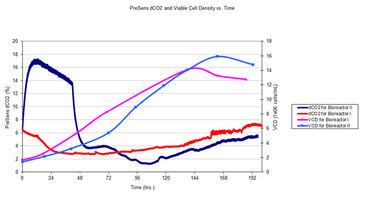
Viable cell density (VCD) was measured off-line by the trypan blue exclusion assay on a Vi-Cell® Cell Viability Analyzer (Beckman Coulter, Inc., Brea, CA). Time-stamped samples were analyzed immediately following extraction from bioreactors, 2 to 3 times daily, on average.
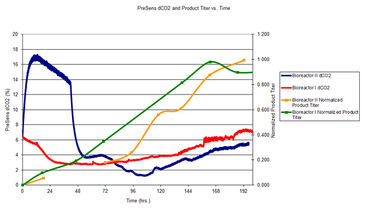
Product titer was determined using a semi-automated gel electrophoresis instrument P200 ScreenTape® System (Lab 901 Limited, Loanhead, UK). Samples for bioreactor run I and II were prepared under non-reducing conditions using reagents and recommendations supplied by Lab901.
Pre-calibrated CO2 sensor patches (PreSens) were adhered with Kwik-Sil silicone (WPI Inc., Sarasota, FL.) onto the bottom of a 12 mm polycarbonate rod designed to fit a standard 12 mm Applikon compression fitting. The pCO2 mini (Fig. 1) was used to monitor changes in dCO2 in the media throughout the bioreactor run with sampling intervals of 30 seconds.
Effect of dCO2 on Cell Culture
In the following research bioreactor runs, the first 48 hours between bioreactor runs I and II exhibited substantial difference in dissolved carbon dioxide levels due to calibration problems with the electrochemical pH probe in bioreactor II. The controller for bioreactor II compensated for the erroneous pH readings by increasing dissolved carbon dioxide levels. By the end of the first day the difference in dCO2 percent between bioreactor II and I was three fold. The high dCO2 had a negative impact on both viable cell density (VCD) and product titer. Even though both bioreactors were seeded at similar initial cell densities, a substantial growth lag was observed for bioreactor II relative to bioreactor I for both VCD (Fig. 2) and product titer (Fig. 3).
Conclusion
Monitoring the percent dCO2 can be valuable in determining root cause for unexpected trends in cell viability and product titer throughout a bioreactor run. Logged percent dCO2 data has been shown here to be helpful in improving the performance of subsequent bioreactor runs by both adjusting process parameters and selecting reliable in-line process sensors.