Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Monitoring Oxygen Dynamics at the Sediment-Water Interface
Non-Invasive Measurements at Distinct Sites in SensorVials

Marion Köster
Ernst-Moritz-Arndt-Universität Greifswald, Institut für Mikrobiologie, Mikrobielle Ökologie, Germany
In marine ecosystems processes at the sediment-water interface are influenced by the presence of an active benthic community living in the uppermost millimeters of the sediment. In coastal zones this benthic compartment is highly active and diverse, including highly productive microalgae, bacteria and protozoans as well as small invertebrates. Photosynthesizing (oxygen-producing) and respiring (oxygen-consuming) activity of the benthic community are key processes that contribute essentially to the temporal and spatial variability of oxygen concentrations at the sediment-water interface. In this study SensorVials with integrated oxygen sensor stripes have been used to investigate oxygen distributions. With the OXY-4 mini oxygen meter measurements were taken at three different positions in the interface zone.
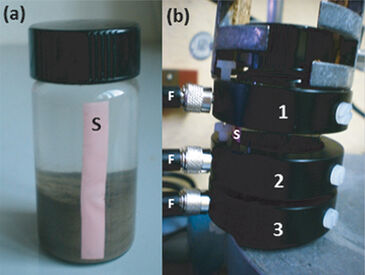
Since the 1980s inserting oxygen microelectrodes and micro-optrodes with tip diameters of less than 50 µm has been an increasingly used method in laboratory and field work to estimate rates of benthic oxygen production and consumption at the sediment surface (e. g. Köster et al., 2008). This invasive technique of microprofiling oxygen measurements can cause changes in the distribution of oxygen concentration and micro-scale distortion in sediment stratification. Compared to this non-invasive and high-resolution oxygen measuring techniques are challenging to estimate the spatial and temporal variability in oxygen concentrations at the sediment surface. The PreSens oxygen sensor stripe is a fluorescence-based optical sensor (type PSt3) that is integrated in experimental containers (Fig. 2 a) and enables the continuous and non-invasive measurement of oxygen at different sites. Vial Adapter rings, each equipped with a hole to insert a polymer optical fiber (POF) and read out the oxygen concentrations, are positioned at distinct sites on the sensor stripe (Fig. 2 b) corresponding to different regions of interest in the environmental sample. In our preliminary study the suitability of the PreSens SensorVial with integrated sensor stripe was tested for monitoring oxygen dynamics at the sediment-water interface of coastal sediments non-invasively. SensorVials were applied in time-course experiments for the simultaneous measurement of the oxygen availability in different depth zones of the sample.
Materials & Methods
Mini-sediment columns of estuarine surface sediments (Rassower Strom, 54°33,657´N, 13°12,657´E, water depth 3.5 m) with microphytobenthic colonization were transferred into 20 mL glass containers that were equipped with a vertical oriented sensor stripe (length of 4 cm, width of 5 mm, Fig. 2). 10 mL of oxygen-saturated, 0.2 µm filtered brackish water was added before the container was closed. Three adapter rings (Vial Adapter, height of 10 mm, hole diameter for POF insertion approx. 3 mm) were exactly fastened by screws at distinct positions along the sensor stripe. Three positions were selected: the sediment surface (0 - 2 mm), a sediment depth of approx. 1 cm, and the overlying water above approx. 2 cm of the sediment surface. Fluorescence signals were transferred via polymer optical fibers to a 4-channel oxygen meter (OXY-4 mini). Data were recorded every 5 min (OXY-4-v2_30FB software). Experimental vials were incubated under light and dark conditions (12 h : 12 h) at 21 °C.
Time-series Experiments
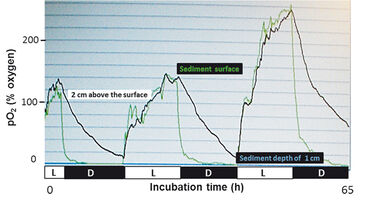
Figure 4 clearly shows fluctuations in dissolved oxygen concentrations at the sediment surface during light and dark incubation (green curve). Oxygenic photosynthetic activity of benthic microalgae caused a continuous increase in pO2 during a 12 h light period. The uppermost 2 mm of the sediment surface were oxygen oversaturated at the end of the light incubation. In the 12 h dark period, intense aerobic respiration activity of the benthic community led to a pronounced decrease from oxygen oversaturation levels to values less than 20 % and 40 % pO2 respectively, within only about 1 h; thereafter oxygen consumption rates were drastically reduced under suboxic incubation. It was of interest that the oxygen producing capability of benthic microalgae exposed to extremely low oxygen concentrations (< 20 % pO2) for 10 h during dark incubation was not negatively influenced in the subsequent light period. Sediments at a depth of 1 cm remained continuously anoxic (pO2 < 1 %) during the total incubation time. Although the water overlying the sediment was not continuously stirred, moving activity of small invertebrates at the sediment-water interface caused the water column to be mixed in the light whereas reduced moving activity in the dark resulted in a slower decrease of oxygen concentrations.
Validation and Future Modifications
Our preliminary results show that the PreSens SensorVials might be suitable tools for measuring oxygen simultaneously in different zones of environmental samples in time-course experiments. It is advantageous that the oxygen measuring sites can be freely selected (positioned) according to the microzones of interest (e. g. sediment-water interface). The integrated sensor stripes and Vial Adapter rings can be easily adjusted to special requirements of environmental samples. In this preliminary study, the number and minimum distance of oxygen measuring positions on the sensor stripe was limited to three positions and 1 cm, respectively. For future investigations, adapter rings of smaller size (height < 10 mm) and / or larger experimental containers with larger sensor stripes are desirable. In addition, sediment sampling cylinders with integrated sensors could simultaneously serve as incubation vials for online monitoring of temporal and spatial fluctuations in oxygen under defined laboratory conditions.
References
Köster, M., Wardenga, R., and Blume, M. (2008) Microscale investigations of microbial communities in coastal sediments. Mar. Ecol. 29, 89 - 105.


