Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Recording Spatial Patterns of Oxygen Consumption in Individual Corals
Assessing Coral Health Under a Changing Climate with VisiSens
Hollie M. Putnam, Denise M. Yost, Ruth D. Gates
Hawaii Institute of Marine Biology, University of Hawaii, Kaneohe, USA
The calcified skeleton of corals makes it difficult to take biological measurements. The VisiSens imaging system was applied to measure oxygen consumption patterns in individual corals immediately after settlement and initiation of calcification. Furthermore, the difference in oxygen consumption between corals exposed to ambient or low pH conditions, which mimic future ocean acidification, was investigated. After as early as two minutes of dark respiration an oxygen gradient across the cross section of the coral spat could be detected with VisiSens. Exposed to low pH, the oxygen consumption of the corals increased compared to measurements in ambient seawater. The VisiSens system provided excellent spatial resolution, and allowed oxygen mapping across all tissue compartments of the corals simultaneously.
As ecosystem engineers, reef building corals create the structural framework of coral reefs. The link between high rates of coral calcification that frame these ecosystems and the presence of photosynthetic endosymbionts in the genus Symbiodinium has been known for over a half century. It is also known that the oxygen production of the symbionts can produce reactive oxygen species that drive coral bleaching, which can cause rapid mass mortality of corals, a serious issue on coral reefs today. In addition increased CO2 in the atmosphere, which is taken up by the surface oceans changes the seawater chemistry, resulting in a decline in pH, or ocean acidification, and drives declining coral calcification. We are currently using confocal microscopy and microCT to image corals in an effort to understand patterns of interaction of the symbionts, coral tissue, metabolism, and calcification. We are currently limited by technology that would allow us to visualize and measure patterns of oxygen production for spatial patterning to overlay against other biological measures. Here we tested the VisiSens oxygen imaging system to increase our understanding of host symbiont interactions, how this may change under environmental stress, and the implications for coral health and persistence under a changing climate. Spatial patterns of oxygen consumption from individual corals immediately following settlement and initiation of calcification, as well as the hypothesis that there is no difference in oxygen consumption between corals exposed to ambient pH and low pH conditions, which mimic future ocean acidification, were investigated.
Materials & Methods
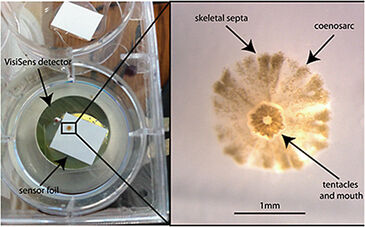
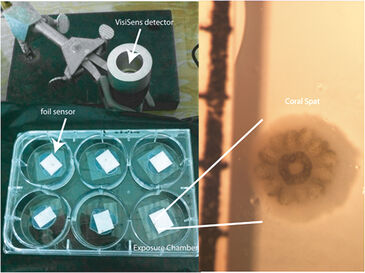
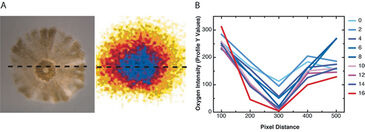
Small chambers were constructed for larval exposure (120 mm2) using the sensor foil and a silicon glue barrier around the foil on the bottom of each circular well (35 mm diameter) in a 6-well plate. 100 µL of treatment water was added to each chamber to cover the newly settled coral (Fig. 1). The VisiSens detector unit was mounted vertically using a clampstand and clamp, so that the bottom of the well and therefore the sensor foil, was in direct and flush contact with the detector face. Coral larvae were collected from the brooding coral Pocillopora damicornis (lace coral) on the fringing reefs of Kaneohe Bay, Hawaii, and placed in 500 mL beakers of 0.2 µm filtered seawater in an incubator at 26 °C with irradiance of approx. 100 µmol photons m-2s-1 of photosynthetically active radiation (PAR). Coral larvae were held for two days and then offered square plastic settlement tiles (44 mm2). Larvae settlement was checked after 2 days and metamorphosed and settled larvae (spat) were used for the experiment. To describe the spatial gradient of oxygen consumption across a cross-section of the coral spat oxygen measurements were carried out in the small chambers on the sensor foils described above. The profiles of live intensity were analyzed every 100 pixels across the row transecting the middle of the polyp (Fig. 3A) over the course of 16 min of oxygen measured in the dark (Fig. 3B). To test the effect of ocean acidification on oxygen consumption, coral spat were placed in the chambers along with 100 µL of seawater at one of two pH conditions resulting from changing the CO2 content or total dissolved inorganic carbon (DIC) at a constant total alkalinity (2190 µmol kg sw-1). Ambient pH was 7.906 ± 0.004 and low pH was 7.572 ± 0.047 (both are mean ± stdev). Coral spat were held in the chamber on the sensor foil in the dark for 15 min. Percent oxygen was calculated by using a two point calibration to 0 and 100 % O2 solutions. The sensor foil was calibrated with a drop of the 0% O2 solution made of sodium sulfite in seawater and background ambient air (100 %).
Oxygen Consumption in Corals
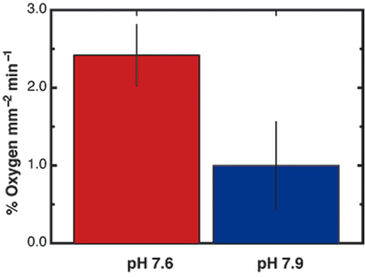
Individual coral spat (approx. 1.5 mm2) respired in the dark at levels detectable by the VisiSens system (Fig. 3A). There was enough resolution after as early as 2 min. of dark respiration to detect a gradient of O2 across the cross-section of the coral spat (Fig. 3B). This analysis of the gradient revealed that O2 consumption was greatest in the center of the organism corresponding to the area surrounding the tentacles and mouth (Fig. 1 and 3), where the tissues are the thickest, and declined towards the edge. The percent O2 consumption in the dark increased following exposure to low pH conditions, in comparison with ambient seawater pH (t = 3.52, p = 0.0293, see Fig. 4).
Conclusion
A difficulty of studying adult reef building corals with a complex morphology is driven by the presence of the calcified skeleton, which precludes flat and uniform contact with the coral surface for biological measurements. In addition, the biological performance of coral tissue is greatly influenced by flow and boundary layer, which in part control the diffusion and gas exchange with the surrounding seawater. We applied the VisiSens imaging system to newly settled coral spat, and found the biology well matched to the technology in terms of the flatness of the foil surface. Oxygen measurements with VisiSens provided excellent spatial resolution, as well as an overall mapping of oxygen consumption by the coral across all tissue compartments simultaneously. The VisiSens system is a powerful tool for understanding the spatial and temporal patterning of oxygen consumption of organisms in situ. To our knowledge, this is the first technology to give micro-scale resolution simultaneous with spatial information. The most commonly used approaches in coral biology include microelectrodes and sealed chamber respirometry. These methods give information at two different ends of the spectrum resulting in single point and whole organism values respectively. The power of the VisiSens system stems from its ability to cover simultaneous the micro scale, as well as, in our case, the whole organism. Pixel level resolution on the order of µm provides the ability to examine the oxygen consumption overlaid on the density of the symbionts in the tissue, as well as the thickness of the tissue itself. Further application of this technology to corals and technological advancements to increase the utility of the system to address questions in morphologically complex organisms, such as branching adult corals will likely bear extremely fruitful results in the future.