Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Cell Growth and Recombinant Protein Production in Small-Scale Culture Vessels
Optical oxygen sensor spots help optimizing culture conditions
V. Jäger1, G. Apati1,2, and N. Konisch1
1Recombinant Gen Expression Group, Helmholtz Centre for Infection Research, Braunschweig, Germany
2Universidade da Região de Joinville, Brazil
Optical measurement of oxygen allows the introduction of small sensor spots into the culture vessels. As many of the conventional sub-bioreactor scale culture vessels are not compatible with polarographic oxygen probes, this technology is a valuable tool for online monitoring in smaller scale cultures. PreSens oxygen sensor spots - used together with the 4-channel oxygen meter OXY-4 mini - have been introduced in Erlenmeyer and Fernbach shake flasks, and in spinner flasks. Measuring oxygen concentrations in cultures of either Sf21 and High Five™ insect cells in combination with the baculovirus expression vector system gave important information on optimal filling volumes in the small-scale vessels and helped identifying metabolic cell activity during cultivation.
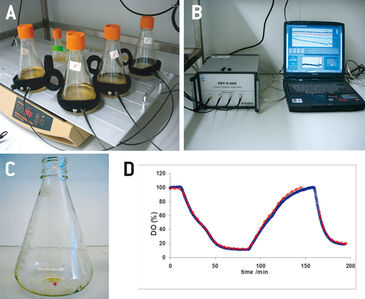
Conventional polarographic oxygen probes, which are usually used for process monitoring of animal cells in stirred tank bioreactors, are very difficult to apply in smaller scale cultivation vessels. Due to their size and only allowing invasive measurements, they cannot be used in vessels without an adequate port, like e. g. in spinner or shake flasks. Optical sensors on the other hand are ideal for oxygen monitoring in these small-scale cell cultivations. Being not bigger than a few millimeters the chemical optical sensor spots can be integrated inside the cultivation vessels, and the sensor response can be read from the outside - through the transparent vessel wall - via optical fiber. This non-invasive approach not only allows easy oxygen monitoring in smaller scale cell cultures, but also reduces the risk of contamination. In this study the PreSens oxygen sensor spots together with the OXY-4 mini - a 4-channel oxygen meter - have been applied for process optimization experiments in sub-bioreactor scale culture vessels, i. e. Erlenmeyer and Fernbach flasks, as well as spinner flasks. Cultures inside such vessels are usually aerated via the surface of the culture medium. Oxygen supply and with it metabolic activity and growth of the cells can be influenced by e. g. changing the rotation speed or introducing baffles in the vessels. However, this exposes the cells to higher mechanical stress, and with higher filling volumes increase of oxygen transfer is limited. Therefore, it is of major importance to monitor oxygen concentrations in small-scale cultivation vessels. Here, the applicability of optical oxygen monitoring is demonstrated in cultures of Sf21 and High Five™ insect cells in combination with the baculovirus expression vector system.
Materials & Methods
Erlenmeyer flasks (125 mL, 500 mL, and 1000 mL) and Fernbach type flasks (3000 mL) with membrane caps (Corning), as well as Techne spinner flasks (nominal filling volume of 125 mL, or 500 mL) were equipped with oxygen sensor spots (SP-PSt3, PreSens) and used for various optimization experiments. Optical fibers connected to a 4-channel oxygen meter (OXY-4 mini, PreSens) were attached to the flasks with Velcro® strips (ARC, PreSens) opposite the sensor spots, for signal read out (Fig. 1 A+B). In a preliminary test the oxygen reading of the optical sensor spots was compared to measurements with a polarographic oxygen probe (Mettler Toledo). IPLB-Sf21 AE and High Five™ cells were grown in Ex-Cell 420 medium (SAFC Biosciences). In addition to the online oxygen monitoring cell numbers (hemocytometer, tryptan blue exclusion), cell size distributrion (CASY cell counter TTC, Roche), glucose and L-lactate concentrations (YSI 2700 analyzer), GFP fluorescence (Guava EasyCyte flow cytometer), and PIGF-1 (sandwich ELISA) were determined and results were compared to oxygen levels [1].
Oxygen Monitoring in Small-Scale Cell Culture
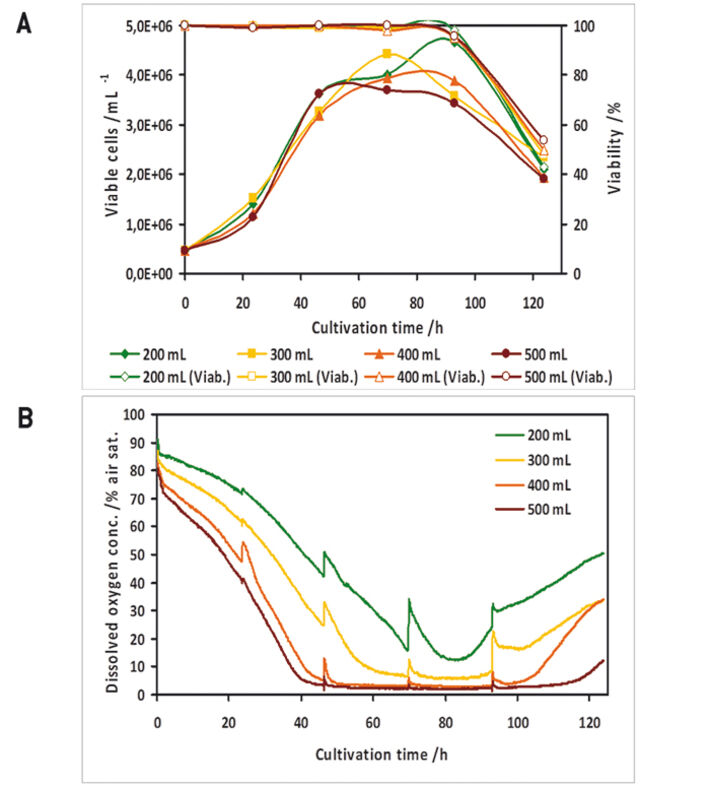
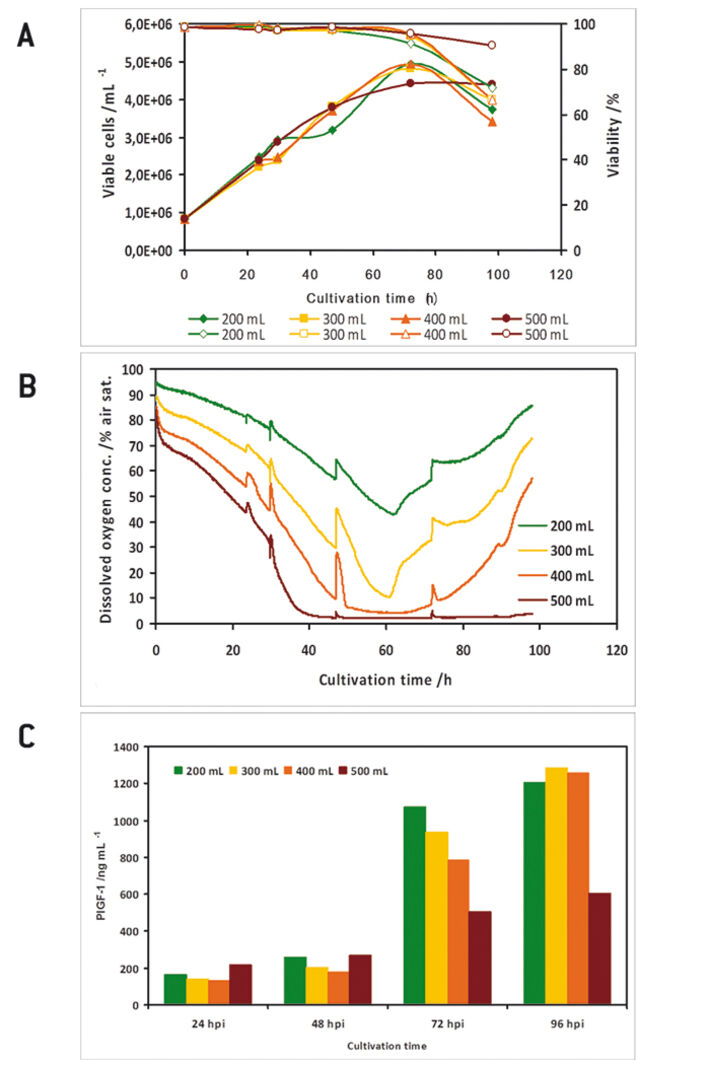
In a preliminary test the oxygen probes inside the flasks showed good accordance to measurements with a polarographic oxygen probe (Fig. 1 D). All experiments were performed with two parallel cultures, with one culture lacking an oxygen sensor, as in this study there were only four channels available for sensor read-out. High Five™ insect cells were grown at different filling volumes in 1000 mL shake flasks. Oxygen limitation occured in the cultures with higher filling volumes (Fig. 2 B). When comparing the growth kinetics of the cultures with the oxygen readings, oxygen limited cultures showed reduced maximum cell density (Fig. 2 A). Analysis results of growth and expression kinetics of baculovirus infected IPLB-Sf21 AE in 1000 mL shake flasks revealed that oxygen limited conditions (filling volumes of 400 and 500 mL) had only a slight effect on Sf21 cell growth (Fig. 3) but recombinant protein production was either retarded or generally reduced. However, cell viability could be maintained better under oxygen-limited conditions. This may have been caused by an inhibition of the viral replication cycle. Here, the online oxygen measurements allowed identifying the current phase of baculovirus infection and revealed possible process limitations. Further experiments were carried out in 125 mL shake flask (data not shown).
Conclusion
The optical oxygen sensors showed very good long-term stability. The sensors were in use repeatedly for more than one year (data not shown). The technology proved to be valuable for process optimization experiments such as identifying suitable filling volumes for recombinant protein expression in spinner or shake flasks. In addition online measurements of oxygen concentrations can be used to senitively estimate metabolic cell activities via the cellular uptake of oxygen thus allowing a predicition of the performance of individual expression experiments.
Application note adapted from
[1] V. Jäger, G. Apati, N. Konisch, scientific poster, 21st ESACT-Meeting, Dublin 2009: Optimisation of cell growth and recombinant protein production in small-scale culture vessels by using optical sensors for on-line measurement of dissolved oxygen.


