Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Characterization of Microbial Growth Profiles
Online detection of oxygen and pH during culture in shake flasks
Konstantin Schneider1, Verena Schütz1, Gernot Thomas John2 and Elmar Heinzle1
1Biochemical Engineering Institute, Saarland University, 66123 Saarbrücken, Germany
2PreSens Precision Sensing GmbH, 93053 Regensburg, Germany
Shake flasks are the most commonly used cultivation systems for process and media optimization, screening for new products and strains as well as for the supply of inoculum for bioreactor cultivation. Oxygen limitation is one of the major though often neglected problems in aerobic shake flask cultivation. The new technology of the SFR Shake Flask Reader is an ideal tool for non-invasive monitoring of the two culture parameters oxygen and pH, and in addition reveals a detailed picture of growth performance and profiles.
To ensure reproducible aerobic cultivations one has to avoid oxygen limitation which would lead to significant changes in central metabolism. Similarly pH changes would also effect the cellular metabolism. Though a series of studies has been carried out to characterize oxygen transfer in shake flasks, various factors lead to changes in oxygen mass transfer that are very difficult to predict. Among these are geometry of usually hand-made baffles and production of surface active compounds. Online monitoring of dissolved oxygen concentration (DO) and pH would also allow the control of these generally important parameters in shake flasks. We use the SFR (www.presens.de/SFR) for our investigations. This device can be applied for the parallel optical online measurement of pH and DO in up to nine shake flasks equipped with the corresponding sensors in a conventional shaker and allows wireless data transmission to a personal computer.
Reproducibility Test During E. coli Cultivation
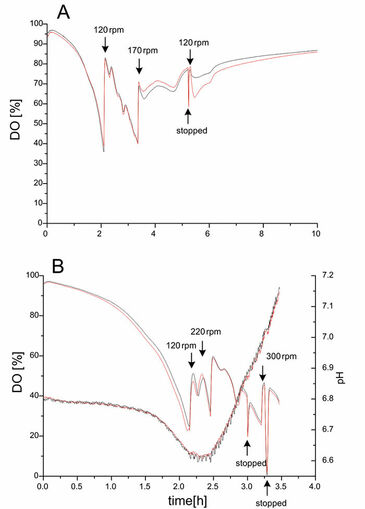
To test the reproducibility of the opto-sensoric measurement system, E.coli was cultivated in baffled glass and unbaffled plastic shake flasks equipped with an oxygen sensor and, in case of the plastic flask, with an additional pH sensor. In both cases shake flasks with similar kLa values were selected. Cultivations were carried out in duplicates at 37 °C and at an initial shaking frequency of 90 rpm. During the cultivation of E.coli DH5a in baffled glass shake flasks an accelerating decline of DO could be observed after inoculation with an optical density (OD 600 nm) of 0.1 (Fig. 1A). After 2 h of cultivation the shaking rate was increased to 120 rpm to avoid oxygen limitation of the culture. After 5.5 h the shaking rate was increased once more to 170 rpm. In the first part ranging from 0 to 2 h exponential growth could be observed, followed by a second phase where different substrates, available in the used LB medium, were consumed. Rearrangement of the cellular metabolism could be observed between 2 and 5 h, where DO concentrations showed some distinct peaks. After 5 h the shaking frequency was reset to 120 rpm. Because of the gradually decreasing respiratory activity of the culture, DO was slowly increasing. The profiles of DO and pH of the E. coli DH5a cultivation in unbaffled shake flasks is shown in Fig. 1B. It is obvious that these flasks were not so well suited for the aerobic cultivation of fast growing E. coli because the oxygen supply was insufficient.
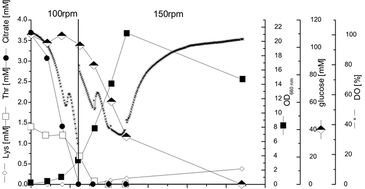
Metabolic Events During Cultivation of C. glutamicum
Cultivation of C. glutamicum was carried out in baffled 250 ml shake flasks equipped with an oxygen sensor at 30 °C and a shaking frequency of 100 rpm. Mean values from duplicates are shown in Fig. 2. Oxygen consumption, growth, uptake of glucose, threonine and citrate as well as lysine production were monitored (Fig. 2). For the investigation of the reproducibility of measurements shake flasks with similar kLa values were used for parallel experiments. Resulting standard deviations of DO were below 3 % showing the high reproducibility of the cultures and the measurement. The cultivation showed a typical two-phase profile with diauxic growth behavior, first primarily consuming citrate and then glucose as major carbon sources. The shift in metabolism from citrate to glucose as carbon source could be precisely determined using the short increase in DO concentration. After 5.5 h the shaking frequency was increased again to 150 rpm due to the high oxygen demand.
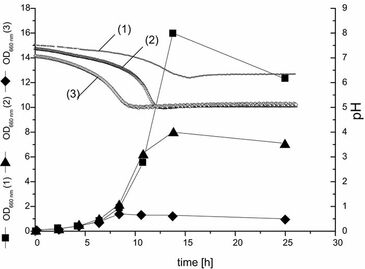
Minimization of Buffer Requirements Using Online pH Measurement
During media optimization, particularly for industrial production, it is mandatory to determine the minimal buffer requirement for an organism. As example C. glutamicum lysCfbr was grown on defined medium with different concentrations of phosphate buffer and pH was monitored online. Cultivation was carried out using 250 ml unbaffled plastic shake flasks equipped with a pH and an oxygen sensor. Cultivation profiles are shown in Fig. 3. Phosphate concentrations of 32 mM and 6 mM were clearly limiting as the OD and DO courses in Fig. 3 show.
Conclusion
The new SFR system provides accurate measurement of DO and pH in shake flask cultures and therefore improves the quality and reliability of cultivations considerably. As the measured data is available online, DO could be controlled using mixtures of air, oxygen and nitrogen. pH could be controlled by the automatic addition of alkali or acid. If kLa is determined, the oxygen uptake rate be determined online during cultivations. A big advantage is the immediate visualization of substrate or oxygen limitation of the culture which is an important factor when using shake flasks as screening device for aerobic processes. DO measurement also gives an online indication of diauxic effects. Online monitoring of DO does of course not eliminate the limitation in oxygen transfer that is observed in shake flasks, but permits control whether oxygen is limiting or not. Many quantitative studies in systems biology are carried out in shake flasks instead of bioreactors. Since shake flasks usually have lower oxygen transfer rates than stirred bioreactors, it is even more important to monitor DO concentration during , e. g., mutant studies to avoid undiscovered limitations that may create large bias and biological misinterpretations in such studies.
Application note adapted from
Schneider et al., 2009, Optical device for parallel online measurement of dissolved oxygen and pH in shake flask cultures. Bioprocess Biosyst Eng DOI 10.1007/s00449-009-0367-0