Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Exchange of O2 and CO2 in the Capillary Fringe of a Porous Medium
A laboratory study testing the VisiSens imaging systems
Christina Haberer1, Massimo Rolle1,2, and Peter Grathwohl1
1Department of Geosciences, University of Tübingen, Germany
2Department of Civil and Environmental Engineering, Stanford University, USA
Diffusion experiments were performed in a quartz sand packing to study the effect of evaporative conditions on mass transfer of oxygen and CO2 across the interface between the unsaturated and the saturated zone. In the experiments, oxygen diffused from the atmosphere into the oxygen-depleted aqueous phase, whereas CO2 was transferred in the opposite direction. We applied the VisiSens™ imaging systems for oxygen and CO2 to map the 2D distribution of these volatile compounds over time. Recording the time-dependent concentration distribution provided insights into the propagation of the diffusion fronts under evaporative conditions. Quantifying the dynamics of oxygen and CO2 and their exchange between different environmental compartments is critical to improve the understanding of the biogeochemical cycles of oxygen and carbon and to assess the impact of forcing factors (e. g. climate change, land use etc.) on groundwater quality.
The coupling between the atmosphere and the subsurface is still poorly understood, and it is extremely challenging to provide a quantitative description of the fluxes of energy and matter exchange between these two compartments. Mass transfer of contaminants and nutrients between the atmosphere and the subsurface occurs at the interface between the unsaturated zone and the underlying groundwater. Few studies have addressed the quantification of such mass fluxes in controlled experimental conditions, in particular under the influence of evaporation. For comparably shallow unsaturated zones without strong microbial activity, gas diffusion is so quick that the partial pressure of volatile compounds can be well approximated by the partial pressure in the free atmosphere. This is different in the underlying aquifer, where diffusion coefficients are by orders of magnitude smaller. The weak vertical mixing in the aqueous phase results in abrupt changes in concentration between the water table and the upper limit of the capillary fringe, which indicate that mass transfer is limited on the water-side. Evaporation affects mass transfer of volatile compounds across the unsaturated/saturated interface as it results in the lowering of this mass transfer boundary over time. With increasing evaporation rate, we expect that the rate of mass transfer is enhanced compared to the case, in which the water level is static. In this study we performed well-controlled diffusion experiments and applied the VisiSens™ imaging systems to investigate mass transfer of oxygen and CO2 between the unsaturated and the saturated zones under evaporative boundary conditions.
Materials & Methods
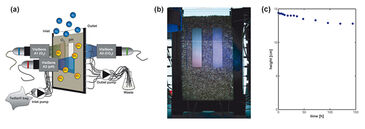
Mass transfer of oxygen and CO2 across the capillary fringe was investigated using the experimental set-up shown in Fig. 1. The diffusion cell had inner dimensions of 10.5 x 19 x 0.5 cm (L x H x W) and was filled with quartz sand (Märkische Kies- und Kalksandsteinwerke GmbH, Germany) with a grain diameter of 1.0 - 1.6 mm. Before starting the experiment, we operated the diffusion cell as a flow-through system to obtain uniform concentrations of oxygen and CO2 within the fully water-saturated zone. Through hollow needles (outer diameter 1.2 mm), a NaCl-solution (ionic strength 100 mM) was injected using a high-precision peristaltic pump (IPC 24, ISMATEC, Switzerland). The NaCl-solution had been flushed with a CO2-containing gas mixture and was then stored in a gastight Tedlar® gas sampling bag. After steady state was reached, the horizontal flow was stopped and the diffusion experiment was started. Oxygen diffused from the atmosphere downwards into the oxygen-depleted aqueous phase, whereas CO2, initially present at high concentration in the saturated zone, diffused upwards. The degassing of CO2 was also expected to increase the initially low pH in the saturated zone (pH 5.13, determined for an effluent sample before the start of the experiment). We mapped the 2D distribution of oxygen and CO2 across the unsaturated/saturated interface using the VisiSens™ imaging systems A1 and A3. The planar sensor foils (SF-RPSu4 and SF-CD1R) with dimensions of 2 cm x 8 cm were glued onto the inner walls of the flow-through chamber (Fig. 1a). We also determined the 2D pH distribution using an additional optrode (VisiSens™ A2 with SF-HP5R, 2 cm x 8 cm). An adapter tubus was mounted on each VisiSens™ camera allowing to depict an area of 2.4 cm x 1.9 cm when taking pictures. By taking several continuous pictures at different points in time the whole optrode was covered. Experiments were performed in a temperature-controlled room at 25 °C. In the experiments water was allowed to evaporate from the porous medium, resulting in a lowering of the capillary fringe over time. We visually determined the decrease fo the upper limit of the capillary fringe as a function of time using a scale that was fixed on one of the glass panes.
Results
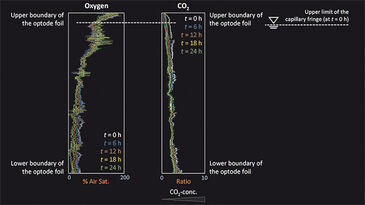
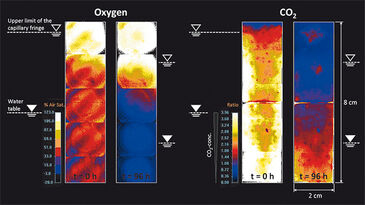
Figure 1c depicts the location of the capillary fringe´s upper limit during the experiment. A drop by 1.7 cm over 144 h was observed, which results in a linear decrease rate of 0.12 mm h-1 assuming constant evaporation. The 2D distribution of oxygen and CO2 at the beginning of the experiment (t = 0 h) and at t = 96 h is shown in Fig. 2. In a separate experiment the capillary fringe height was previously determined to be 4 cm. Due to the high water content within the capillary fringe we observed a concentration gradient at its upper limit for both oxygen and CO2. Initially (i. e., at t = 0 h), the concentration gradients were very steep, however, they became flatter over time as the oxygen front progpagated deeper into the saturated porous medium and, at the time, CO2 progressively degassed from the saturated zone to the atmosphere. The same effect is shown in Fig. 3, which presents the measured vertical profiles of oxygen and CO2 between t = 0 h and t = 24 h, determined at 6-h time intervals, after the beginning of the experiment. Especially for CO2 the typical time-dependent behavior of diffusion can be observed in the upper part of the vertical profile.
Assuming a rapid change in water content at the upper limit of the capillary fringe and the location of the capillary finge´s upper limit does not change over time, mass transfer across the unsaturated/saturated interface is described by the analytical solution of the governing diffusion equation: c = (c0 - cbg) erfc (z / 2√Dpt) + cbg, in which c [M L-3] is the concentration determined at time t [T], and at the vertical location, z [L], along the profile. c0 [M L-3] represents the concentration at the unsaturated/ saturated interface (z = 0 m) and the background concentration within the saturated zone, respectively. The pore diffusion coefficient, Dp [L2 T-1], is given by Dp = Daq / T, where Daq [M2 T-1] is the aqueous diffusion coefficient of the compound considered and T [-] is the tortuosity of the porous medium. By applying the correct boundary conditions, the analytical solution describes the observed behavior for both oxygen and carbon dioxide; a deviant behavior could be due to evaporation. Our measurement results for pH are not shown as the resolution was too low at pH 5.13 and a significant increase in pH could not be detected over time.
Conclusion
We applied the VisiSens™ imaging systems for oxygen and CO2 to evaluate the mass transfer of these volatile compounds across the unsaturated/saturated interface in a porous medium and in the presence of evaporation. The imaging systems allowed us to capture and visualize the dynamics of the concentration gradients of O2 and CO2 during the experiments. Such controlled laboratory experiments are instrumental for understanding the coupling between the atmosphere and the subsurface. Moreover, such experiments are helpful to constrain physically-based numerical models describing those coupled processes at larger field scales and attempting to answer research questions of fundamental relevance such as the evolution of biogeochemical cycles, the transport of volatile contaminants at large scales, and the evolution of groundwater quality in scenarios considering dynamic forcing factors (e. g. climate change, increasing anthropogenic pressure and change of land use).