Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Hypoxic Preconditioning of Human Mesenchymal Stem Cells
Restoring the Differentiation Potential of hMSCs under Hypoxic Conditions
Elias Volkmer1, Booby Cherian Kallukalam1, Josef Maertz1, Sven Otto2, Inga Drosse1, Hans Polzer1, Wolfgang Bocker1, Michael Stengele1, Denitsa Docheva1, Wolf Mutschler1, and Matthias Schieker1
1Experimental Surgery and Regenerative Medicine, Department of Surgery, LMU Munich, Germany; 2Department of Oral and Maxillofacial Surgery, LMU Munich, Germany
In this study the osteogenic differentiation potential of hMSCs under hypoxic conditions of 2 % O2 in comparison to standard tissue culture oxygen atmosphere of 21 % was investigated. Further tests were conducted to determine whether hypoxic preconditioning has an effect on the following osteogenic differentiation of hMSCs and if this pretreatment might be beneficial for the differentiation process. It was found that hMSCs proliferate better when cultured at 2 % O2 but constant hypoxia inhibits osteogenic differentiation. Hypoxic preconditioning of hMSCs prior to osteogenic induction restores osteogenic differentiation of the cells under hypoxic conditions.
Hypoxia might be directly connected to the osteogenic differentiation of bone precursor cells as the reduced oxygen supply after bone fracture triggers the healing process. The 21 % oxygen atmosphere commonly used in cell culture is rather considered a state of hyperoxia and low oxygen tensions seem to be a more physiological milieu for stem cells. Cells used for tissue engineering seeded on a 3D scaffold often are subjected to hypoxic conditions because of the uneven oxygen supply along the 3D construct. As hMSCs might be exposed to low oxygen concentrations in tissue engineering it is of great importance to investigate the effect of hypoxia on their regenerative and differentiation potential. Many studies have already shown that hypoxia has a negative effect on osteogenic differentiation. In this study it first was shown that primary hMSCs as well as fast growing human telomerase reverse transcriptase (hTERT) immortalized hMSCs are exposed to low oxygen concentrations during static 3D culture. For oxygen measurements in the center of the 3D constructs needle-type oxygen microsensors by PreSens were used. In further tests it was investigated whether low oxygen of 2 % would affect proliferation of the hMSCs and if preconditioning hMSCs in hypoxia would restore their differentiation potential.
Materials & Methods
hMSCs at passage 2 (P2) were purchased from Lonza (Verviers, Belgium) and used in all experiments. Cells of a primary donor, and for repeating key experiments hMSCs of two further donors were used. hTERT-immortalized hMSCs, developed in our laboratory, proliferate faster than primary hMSCs and served as a control in all experiments. Derived from a single cell-picked clone of the hTERT-immortalized hMSCs they were named single cell-picked clone 1 (SCP-1). All cells were expanded in minimum essential medium alpha with L-glutamine (Invitrogen, Carlsbad, CA) [1] at 37 °C in 5 % CO2 in a standard cell culture incubator (21 % O2). For static 3D culture cylindrical bovine demineralized bone matrix (Tutogen, Neunkirchen, Germany) scaffolds of 9 mm in diameter and 5 mm in height were seeded with 1 million P8 cells [2]. The cell-scaffold constructs were transferred to a 24-well dish (Nunc, Wiesbaden, Germany) and cultured for 7 days under standard conditions. For oxygen measurement in the 3D cultures needle-type oxygen microsensors (PreSens, Regensburg, Germany) were introduced in the geometric center of the 3D constructs, seeded with either 1 million hMSCs or with 1 million SCP-1 cells. Addition of fresh medium was performed once after 4 days. The effect of hypoxia on hMSCs was tested by keeping the cells in a multigas incubator (MCO-5M, Sanyo, Pfaffenhofen, Germany) that maintained a gas mixture composed of 93 % N2, 5 % CO2, and 2 % O2. For osteogenic differentiation P6 hMSCs were plated in a six-well plate (Nunc) at a density of 300 cells/cm2 after trypsinization. They were cultured under normoxia until subconfluency was reached. Then three of the six wells in each plate were induced to differentiate towards the osteoblastic lineage [3]. Cells were grown in osteogenic differentiation medium [1] and for negative control the remaining three wells were cultured with complete medium. After induction the plates were either incubated in a low-oxygen atmosphere of 2 % O2 for the remaining period of differentiation (hypoxic samples) or they were kept under normoxic conditions (normoxic control samples). Hypoxic preconditioning was realized by expanding P6 hMSCs under hypoxic conditions of 2 % O2 until subconfluency was reached. Osteogenic differentia- tion was then carried out under hypoxia (2 % O2) in the same way as described for the hypoxic samples. A hypoxia detection assay and also a western blot analysis of Hif-1 alpha was performed to proof hypoxic conditions [1]. Hif-1 alpha is part of a complex signaling cascade, induced as cells respond to hypoxic conditions. A water-soluble terazolium salt assay was performed to monitor the cells´ metabolic activity during prolonged exposure to hypoxia and after 21 days of osteogenic differentiation an Alizarin red staining was performed to show calcium depositions [1]. Additionally a RT-PCR assay should show further temporal expression patterns of important osteogenic markers during osteogenic differentiation [1].
Hypoxia and Preconditioning
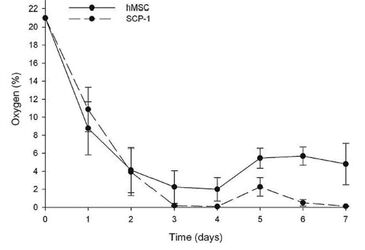
Oxygen measurements in static 3D culture of hMSCs and SCP-1 cells over 7 days show that cells cultured this way are exposed to hypoxic conditions. The oxygen concentration in the central region of hMSC-seeded scaffolds dropped from 21 % O2 at day 1 to around 4 % of oxygen at day 4. After change of medium, the oxygen level rose to approx. 5.7 % where it evened out. The well-growing SCP-1 cells on the other hand consumed oxygen faster, resulting in a decline to 0 % at day 3. Upon addition of fresh medium, the oxygen levels rose shortly to approx. 2 % before they again dropped to 0 % (Fig. 1).
In the 2D experiments Hif-1 alpha protein was detected in the hypoxic samples but not within the normoxic control samples. hMSCs were osteogenically differentiated in the presence of 21 % oxygen and compared with samples cultured at 2 % oxygen atmosphere. Normoxic samples differentiated well as confirmed by a strong Alizarin red staining, whereas the cells under hypoxia differentiated poorly, suggesting a decrease or delay in osteogenic differentiation under low-oxygen conditions. As a control the osteogenic differentiation was monitored measuring the temporal RNA expression patterns of the represen- tative osteogenic markers ALP and OPN. The RT-PCR results showed that the expression level of OPN under normoxia constantly increased from day 5 to 16 over day 21. The expression of ALP was equally high. When differentiating cells under hypoxia the expression of the two markers was clearly reduced at days 16 and 21. On day 5, on the other hand, upregulation of ALP was almost equal under hypoxia whereas OPN expression was even higher in the hypoxic setting compared to the normoxic samples. For testing whether preconditioning hMSCs in hypoxia would improve their differentiation potential under constant hypoxia, cells were cultured in 2 % oxygen atmosphere for 3 days prior to osteogenic differentiation. The results showed that this treatment restored the differentiation capacity as substantiated by a positive Alizarin red staining and the RT-PCR results revealed that hypoxic preconditioning restored both OPN and ALP upregulation upon osteogenic induction at days 16 and 21. A comparison of normoxic, preconditioned hypoxic and non-preconditioned hypoxic samples showed a significantly decreased but sizeable extent of staining within the preconditioned samples compared with normoxic samples, whereas there was literally no detectable Alizarin red staining in samples from the hypoxic set-up. To verify results the key experiments were repeated with cells from two other donors with almost identical outcome.
Conclusion
The results obtained when measuring oxygen in 3D culture with hMSCs and hTERT-immortalized hMSCs suggest that primary hMSCs adapt to the low-oxygen atmosphere better than the SCP-1 cells. One explanation may be that primary hMSCs simply grow slower and thus have more time to slow down proliferation when oxygen levels decrease. This fact has to be kept in mind when using genetically modified stem cells for regenerative medicine. The results support the thesis that preconditioning of hMSCs may improve their potential to differentiate towards the osteoblastic lineage under constant low-oxygen conditions. We assume that the process of osteogenic differentiation is disturbed by changes in oxygen tension, whereas constant conditions support the process of differentiation. This would explain why hMSCs either differentiated under constant normoxia or if cells were grown in hypoxia for 3 days before they were differentiated under hypoxic conditions. Hypoxic preconditioning might be a helpful tool for successful cell-based therapies.
Application note adapted from
[1] Volkmer et al., 2010, Hypoxic Preconditioning of Human Mesenchymal Stem Cells Overcomes Hypoxia-Induced Inhibition of Osteogenic Differentiation, Tissue Eng. Part A vol. 16, pp. 153 - 164
References
[2] Volkmer et al., 2008, Hypoxia in Static and Dynamic 3D Culture Systems for Tissue Engineering of Bone, Tissue Eng. Part A Vol. 14, 1331
[3] Seitz et al., 2007, Influence of in vitro Cultivation on the Integration of Cell-matrix Constructs after Subcutaneous Implantation, Tissue Eng. Vol. 13, 1059