Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Monitoring pO2 in Cell Culture to Improve Directed Differentiation
Visualizing oxygen distributions in 6 well plates with VisiSens A1
S. M. Rolandin1,2, C. Fraker1, V. Manzoli1, C. Villa1,2, A. A. Tomei1, Y. Torrente2, L. Inverardi1, J. Dominguez-Bendala1
1Diabetes Research Institute, University of Miami Miller School of Medicine, USA
2Stem Cell Laboratory, Department of Pathophysiology and Transplantation, Fondazione Ca´Granda IRCCS, Ospedale Maggiore Policlinico, Centro Dino Ferrari, Universita di Milano, IT
Tissue oxygenation during in vitro culture of stem cells is a critical factor. Previous studies have shown that maintaining pO2 during in vitro culture at levels approximating the physiological niche of the target population can significantly improve terminal differentiation. In this study the VisiSens™ oxygen imaging system was applied in a 6 well plate cultured with immortalized C2C12 cells to investigate spatial and temporal changes in oxygen concentrations over the surface of the cell culture. Over the course of the measurement period the oxygen level in the vicinity of the cells decreased. First results show that oxygen concentrations over the cross section of the cell culture can vary significantly over time, and that VisiSens™ is a suitable tool for oxygen monitoring in the stem cell field. Implementing the sensors to monitor culture conditions in real-time might significantly contribute to create reproducible environmental conditions and improve protocol standardization and differentiation outcome.
Efficiently guiding targeted development of stem cells during in vitro culture only had limited success so far. One reason might be the simplicity of in vitro culture systems that cannot equal the complexity of in vivo microenvironments. Because so far the focus was put on molecular variables, current differentiation methods are inefficient and rarely yield fully functional target cells. Recent research in the stem cell field now also concentrates on critical and important physical parameters, like e. g. tensile forces, ionic signaling, and mechanical pressure, as key factors for cell differentiation.
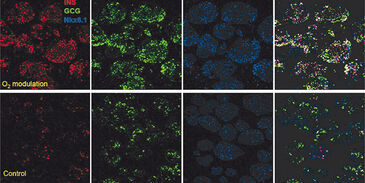
In a previous study we have shown, that tissue oxygenation during in vitro culture is also an important parameter in the specification of endocrine cell types. When the culture is maintained at levels approximating the physiological niche of the target cell population, immunofluorescence results (Fig. 1) show higher expression of insulin and glucagon with improved separation of hormone co-localization compared to standard culture controls.
Tissue oxygenation during in vitro culture depends on factors like cellular oxygen consumption rate (OCR), proliferation / cell death, plating density, and medium depth. Especially OCR and proliferation rates can vary significantly from passage to passage. Such variables, however, are often overlooked and might lead to the variability observed in stem cell culture and differentiation. The use of biosensors for online control of the culture environment could reduce variability in differentiation protocols and improve differentiation standardization.
In this study we tested the VisiSens™ oxygen imaging system for its suitability in online oxygen monitoring of stem cell culture and further application in the stem cell field. It was applied in C2C12 cell culture to get first insights on oxygen distribution at the culture surface.
Materials & Methods
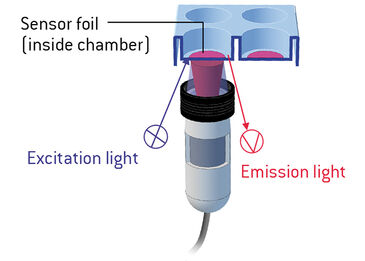
The VisiSens™ oxygen sensor foils (SF-RPSu4, PreSens, Regensburg) were applied inside a 6 well plate. The sensors were calibrated using DMEM Hi suspended with 10 % FBS, 1 % P/S, and L-glutamine for 100 % R/A saturation and 100 mM Sodium Hydrosulfite dissolved in dDH2O for 0 % oxygen saturation. Then C2C12 cells were plated at a density of 1.03 x 105 cells/in2 in 1.6 % w/v alginate solution on top of the sensor foil. Alginate was gelated for 10 minutes with 50 mM CaCl2 solution and then washed adding DMEM Hi for overnight culture. The 6 well plate with the cells and the VisiSens™ detector unit (DU01, PreSens) were placed in a standard humidified incubator with 10 % O2, 5 % CO2. Recording of the images was initiated after five minutes for a duration of 16 hours. After image recording the data and oxygen profiles across the entire sensing surface were analyzed with the VisiSens™ AnalytiCal 1 software.
Oxygen Imaging in Cell Culture
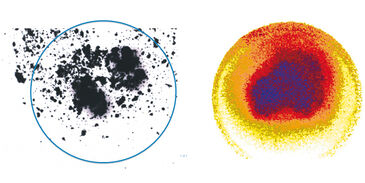
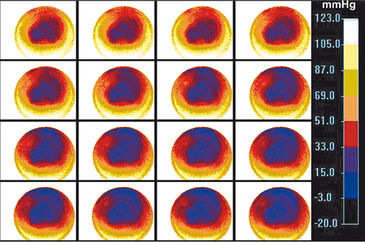
Testing the VisiSens™ oxygen imaging system with the immortalized muscle cell line C2C12 showed the suitability of this device for monitoring oxygen gradients across the cell culture surface over time with high spatial resolution. As depicted in Figure 3 oxygen levels in areas with higher cell density are significantly lower, than in the surrounding medium. Further oxygen decrease was monitored in the center of the culture over the next 80 minutes (Fig. 4). The system allowed to get real-time information about the oxygen levels over a cross section of the cell culture. As the detector unit can easily be applied inside the incubator in controlled environment, the system is ideally suited for cell culture monitoring. Implementing these sensors and the VisiSens™ imaging system for real time culture monitoring could significantly improve the reproducibility of environmental conditions, and with it guiding and optimizing in vitro maturation.
Conclusion
It is clear that oxygen plays a significant role in the differentiation outcome of precursor cells and modulation of this critical environmental factor could result in substantially more efficient stem cell differentiation protocols. The application of VisiSens™ and the fluorescent sensor foils enables online 2D monitoring of oxygen gradients over the cell culture surface, so oxygen levels could be adjusted if necessary. When investigating the role of O2 in pancreatic development, the use of the sensors could generate new experimental strategies to improve the efficiency of existing beta cell differentiation protocols. By characterizing O2 levels encountered by the cells during conventional and modulated oxygen culture and integrating this information in the design of effective beta cell differentiation protocols, clinical translation of stem cell-based approaches for treatment of insulin-dependent diabetes might be accelerated. A custom in vitro culture system where online sensor feedback, like provided by VisiSens™, is used to precisely control and adjust environmental variables, such as pO2, in response to changes in OCR, cellular growth, death and remodeling, could ensure physio-optimal levels, and help overcome variability and shortcomings of current in vitro culturing methods.
Application Note adapted from:
S. M. Rolandin, et al.: Temporal monitoring of pO2 in cell cultures: a potential tool for directed differentiation; scientific poster, World Stem Cell Summit 2012