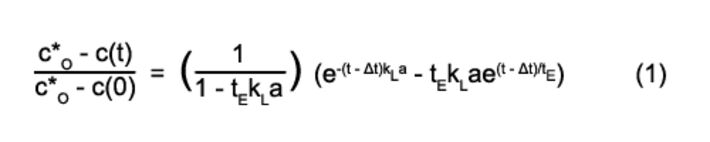Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
New mL-Scale Bioreactor for the Cultivation of Mycelium Forming Microorganisms
Determination of the Volumetric Oxygen Transfer Coefficient (kLa)
Ralf Hortsch1, Ansgar Stratmann2, Dirk Weuster-Botz1
1Institute of Biochemical Engineering, TU Munich, Germany
2W42 Industrial Biotechnology GmbH, Marl, Germany
A new milliliter-scale stirred tank bioreactor was developed for the cultivation of mycelium forming microorganisms. Applying a newly designed one-sided paddle impeller an enhanced surface-to-volume ratio of the liquid phase was generated where oxygen was introduced in the medium via surface aeration. The fast moving liquid lamella efficiently prevented wall growth and foaming in the mycelium forming culture. In the new bioreactors volumetric oxygen transfer coefficients (kLa) > 0.15 s-1 were measured applying chemical optical oxygen microsensors with fast response time.
The number of biotechnological reactions has significantly increased throughout the past years. Therefore, the miniaturization of bioreactors for bioprocess development is of high interest. One important group of industrial production organisms are mycelium and pellet forming microorganisms like actinomycetes or fungi, as - for example - they are able to produce the majority of antibiotics. A bioreactor system for cultivation of mycelium forming microorganisms would have to meet the following requirements: It has to ensure a sufficient gas-liquid mass transfer in viscous media, distribute power uniformly into the reaction medium and prevent wall growth efficiently. Thus a new impeller concept has been developed (Hortsch and Weuster-Botz, 2009; German Patent Application DE 10 2009 056 468.3). Different test and measurements - like volumetric power consumption, maximum local energy dissipation, and reference cultivations at liter-scale - were performed to investigate the usability of the new bioreactor system. As sufficient oxygen supply is a key requirement for most microbial cultivations the oxygen transfer coefficient kLa of the new impeller was measured applying oxygen microsensors by PreSens GmbH, Germany. Integrated chemical optical sensors (PreSens) at the bottom of each bioreactor additionally allowed monitoring the dissolved oxygen content and pH in the cultivation medium.
Materials & Methods
The genetically modified strain Streptomyces tendae W42-0 (W42 Industrial Biotechnology GmbH, Germany) served as a model organism in all experiments, as this actinomycete shows distinctive behavior of filamentous microorganisms. The newly designed single-use reaction vessels without baffles had an inner diameter of about 20 mm. They were equipped with the impeller prototype; chemical optical sensor spots for pH and dissolved oxygen concentration (DO) measurement were attached to the bottom of each reactor. The vessels were designed to fit a bioreaction block described by Weuster-Botz et. al. in 2005 [4] with a maximum of 48 stirred tank reactors. A gas distributor with the 48 impellers mounted on individual axes assured the sterile gas distribution to all 48 stirred tank reactors and served at the same time as sterile barrier. An optosensoric measurement system - applying an oxygen microsensor with a response time between 2 and 3 seconds and the fiber optic oxygen transmitter Microx TX3 (PreSens) - was used to determine the oxygen transfer coefficient in the milliliter-bioreactors. The kLa was measured using the dynamic sulfite method. All measurements were performed in 0.5 M Na2SO4. At the beginning of each measurement the aqueous solution, containing 10-3 M CoSO4 as a catalyst, was saturated with air. A certain amount of a Na2SO3 solution was added to consume all dissolved oxygen until the value dropped to zero. After depletion of the sulfite the saturation phase was monitored and Equation 1 was adapted to the saturation curves with the Levenberg-Marquardt algorithm (Labview 7.4, National Instruments, Austin, Texas) to determine the kLa value:
The oxygen saturation concentration c*O refers to the inlet gas. The response time tE of the oxygen sensor is the time needed to monitor 90 % of a step change in dissolved oxygen concentration. The experimental time t is introduced as a floating parameter by allowing a time lag delta t between the addition of sulfite and its stoichiometric consumption [2].
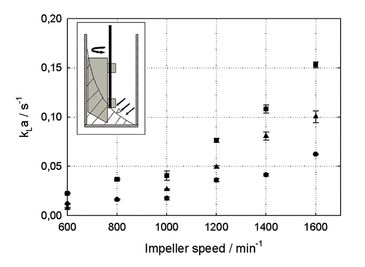
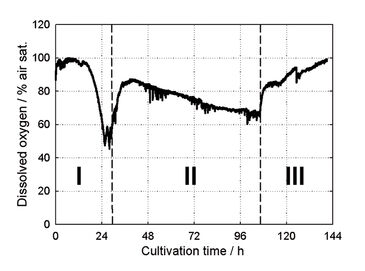
kLa and DO Measurements
Depending on the reactor volume kLa > 0.15 s-1 could be measured (Fig. 1). This kLa is significantly higher than in other surface aerated systems, like e. g. shake flasks, and is within the range of technical stirred tank bioreactors with kLa 0.05 - 0.3 s-1 under normal conditions [3]. The kLa value is dropping with growing reaction volume because of the smaller specific gas/liquid exchange area. With the new paddle an estimated surface-to-volume ratio of 0.4 mm2 mm-3 could be achieved for 8 mL reaction volume and 1,600 min-1 impeller speed; this is about 10fold higher compared to other systems without the paddle. As the specific gas/liquid exchange area is only slightly affected by increasing viscosity in surface aerated processes, the new impeller has many advantages for cultivations with viscous media, like most mycelia cultivations. kLa remains relatively constant in viscous fluids whereas in bubble aerated systems the kLa can drop up to 20 times during cultivation with filamentous microorganisms [1]. During cultivation the dissolved oxygen concentration in the medium was monitored with the integrated chemical optical sensors. This data shows, that at no time an oxygen limitation occurred during bioreactor runs (Fig. 2). The newly developed impeller system guarantees a sufficient oxygen supply for the culture. In Figure 2 the different growth phases of an exemplarily chosen cultivation of Streptomyces tendae in the newly designed milliliter-bioreactors can be determined.
Conclusion
kLa determination and online DO monitoring - together with a number of other tests - showed the usability of the new milliliter-bioreactors operated in a bioreaction block for cultivation of mycelium forming microorganisms and parallel bioprocess development. The measured kLa is within the range of technical stirred tank bioreactors and higher than in other surface aerated systems. Even with increasing viscosity the new impeller can supply enough oxygen to the cultivation. The fast moving liquid lamella efficiently prevents wall growth and foaming in the mycelium forming culture. In prolonged fedbatch culture it has to be taken into account that the oxygen transfer rate decreases with increasing volume in the milliliter-scale bioreactors. Therefore, the impeller speed might have to be increased or the headspace of the reactors could be enriched with oxygen. Online measured state variables like DO and pH can be monitored and controlled in every single reactor with integrated chemical optical sensors. This enables parallel cultivations on the milliliter-scale comparable to the laboratory- and pilotscale.
Application note adapted from
Hortsch et. al., 2010, New Milliliter-Scale Stirred Tank Bioreactors for the Cultivation of Mycelium Forming Microorganisms; Biotech. & Bioeng. Vol. 106, No. 3, pp. 443 - 451
References:
[1] Badino A.C., Facciotti M.C.R., Schmidell W. (2007); Volumetric oxygen transfer coefficients (kLa) in batch cultivations involving non-Newtonian broths. Biochem Eng J 8: 111 - 119.
[2] Linek V., Vacek V., Benes P. (1987); A critical review and experimental verifiction of the correct use of the dynamic method for the determination of oxygen-transfer in aerated agitated vessels to water, electrolyte- solutions and viscous liquids. Chem Eng J 34: 11 - 355.
[3] Van´t Riet K. (1979): Review of measuring methods and results in nonviscous gas-liquid mass transfer in stirred vessels. Ind Eng Chem Process Des Dev 18: 357 - 364.
[4] Weuster-Botz D., Puskeiler R., Kusterer A., Kaufmann K., John G.T., Arnold M. (2005); Methods and milliliter scale devices for high-throughput bioprocess design. Bioprocess Biosys Eng 28: 109-119