Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Oxygen Measurements of Innate Immune Cells in Suspension Cultures
In vitro Monitoring with Fibox 4 and Optical O2 Sensor Spots
Helene Möllerherm, Katja Branitzki-Heinemann, Maren von Köckritz-Blickwede
Department of Physiological Chemistry and Research Center for Emerging Infections and Zoonosis, University of Veterinary Medicine Hannover, Germany
Here, we describe data on the oxygen measurements in suspension cultures of innate immune cells: Primary murine bone marrow-derived mast cells and primary human blood-derived neutrophils. Firstly, we characterized the oxygen level during the long-term differentiation process of bone marrow derived mast cells around 7 % O2 (53 mmHg) reflecting conditions in the intestinal tissue (Part 1, published in [5]). Furthermore, short-term incubation under acute hypoxic conditions (1 % O2, 7 mmHg) of both, neutrophil and mast cell cultures was evaluated for the usage of studies focusing on the host-pathogen interaction (Part 2, published in [6, 7]).
The innate immune cells act as immediate host defense against bacterial infections. Upon infection, tissue resident immune cells as mast cells are activated and release proinflammatory mediators that subsequently lead to recruitment of additional immune cells. Neutrophils are the first innate immune cells that transmigrate to the site of infection and mediate killing of invading pathogens.
Most studies of innate immune cells functions are done in normal tissue culture incubators with standard culture conditions at atmospheric conditions, about 21 % O2, 5 % CO2, 37 °C. But these conditions do not reflect the physiological relevant conditions: In the healthy situation, the physiological oxygen levels depend on the tissue location with e. g. concentrations around 7 % in the intestinal tissue [1]. In case of infection and inflammation, the consumption of oxygen by the pathogens and the transmigrated immune cells lead to hypoxic oxygen levels around or even below 1 % with consequences for cellular functions [2 - 4]. To better understand the function of innate immune cells for development of new therapeutic or prophylactic intervention strategies, studies with innate immune cells need to be performed under physiological relevant conditions. To analyze these functions correctly, oxygen levels during in vitro studies have been monitored in the presented study with an optical oxygen measurement system.
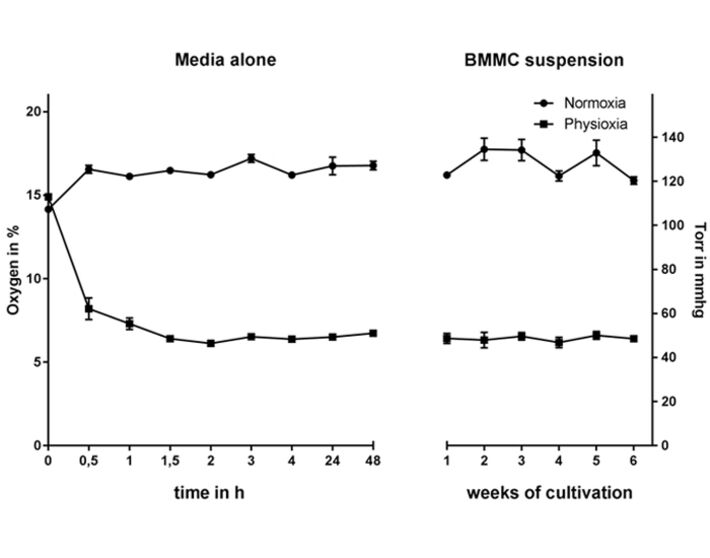
1. Long-term Physiological Incubation (53 mmHg, 7 % O2) of Differentiating Mast Cells
For long-term oxygen measurements, hematopoietic progenitor cells were isolated from the bone marrow of C57BL/6 wildtype mice and differentiated in the presence of interleukin-3 for 39 days (method described in [8]) under physiological oxygen conditions (hypoxia glove box, Coy Laboratory Products, with 7 % O2, 53 mmHg) in comparison to atmospheric oxygen conditions (normal CO2 incubator, BINDER, at atmospheric oxygen level, 21 % O2). The experimental settings of cultivating mast cells under physioxia were monitored over the whole differentiation period using the Fibox 4 and O2 Sensor Spots SP-PSt3 (PreSens).
The oxygen level stabilizes at around 53 mmHg (7 % O2), while the normoxic remains above 120 mmHg (16 % O2) in media alone as well as in the cell suspension (Fig. 1), confirming that MCs do not massively consume oxygen in the culture media during differentiation. To evaluate the time needed for the media to equilibrate to physioxic or normoxic oxygen levels the oxygen content was monitored up to 48 h under hypoxia (without using pre-equilibrated media). The oxygen level equilibrates after 1.5 h and stays constant for up to 48 h (Fig. 1) [5].
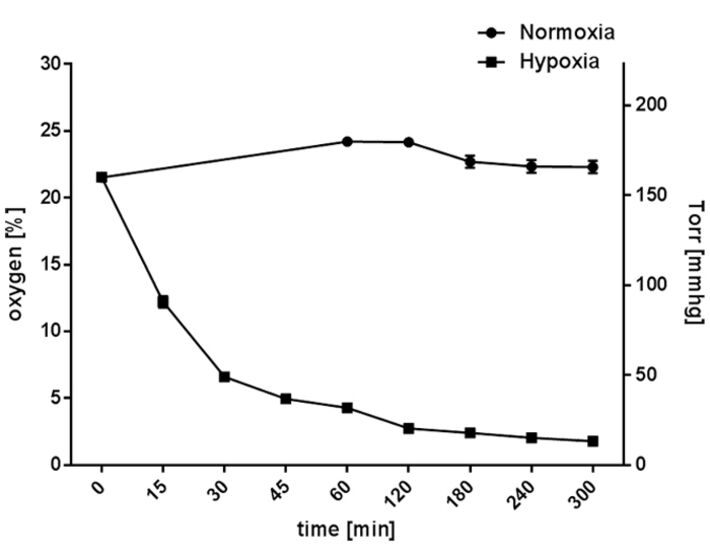
2. Short-term Hypoxic Incubation (7 mmHg, 1 % O2) of Mature Neutrophils and Mast Cells
Freshly isolated human neutrophils derived from healthy human blood donors (methos described in [9]) and differentiated primary murine bone marrow-derived mast cells (method described in [8]) were seeded in 24-well suspension culture plates (Sarstedt, cell culture plate, polystyrene, for suspension cells) and incubated under hypoxic (hypoxia glove box, Coy Laboratory Products, 7 % O2) compared to normoxic (CO2 incubator, BINDER, 159 mmHg, 21 % O2, 5 % CO2) conditions. The oxygen level was measured over a time period of 5 h using the Fibox 4 and O2 Sensor Spots SP-PSt3 (PreSens).
When cultured under normoxia, the neutrophil cultures maintained a constant oxygen level that reflected the atmospheric conditions at around 165 mmHg (22.3 ± 0.46 % O2). Hypoxic incubation decreased the dissolved oxygen level in the culture to less than 37 mmHg (4.9 ± 0.2 % O2) within 45 min (without pre-equilibration of media) and resulted in an oxygen level lower than 13.3 mmHg (1.79 ± 0.03 % O2) within 5 h (Fig. 2). In comparison, mast cell cultures maintained a constant oxygen level around 125 mmHg (16.9 ± 1.2 % O2) under normoxia (Fig. 3). Hypoxic incubation decreased the dissolved oxygen level in the culture media to less than 28 mmHg (3.7 ± 0.7 % O2) within 45 min and resulted in a stable equilibrium lower than 7 mmHg (0.9 ± 0.2 % O2) within 5 h (Fig. 3) [6, 7]. By comparing these oxygen measurements of suspension cell cultures with adherent cell cultures, like epithelial cells grown as attached monolayer [10], distinctly higher oxygen levels are measurable in both neutrophil and mast cell suspension cultures.
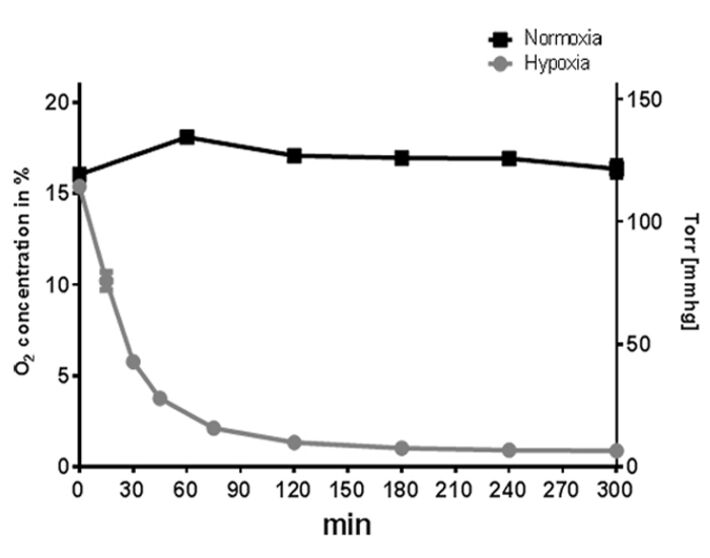
Short Summary and Conclusion
In summary, based on the presented oxygen measurements, we are able to show that suspension cultures of innate immune cells exhibit oxygen levels in the medium that are close to environmental level. That is different to most epithelial cell cultures grown in a monolayer [10, 11]. Thus, our results shown here, confirm that the applied experimental settings, by incubating neutrophils and mast cells under hypoxia, decrease the oxygen level and reflect physiological oxygen conditions that may occur in infected tissue. Based on these experimental setting, using a hypoxia glove box with constant oxygen level, we performed intense in vitro studies that showed that neutrophils and mast cells act differently when cultured under physiological and hypoxic oxygen conditions and highlight the importance of using respective oxygen conditions for cellular studies on innate immune cell functions [5 - 7].
References
[1] Carreau, A. et al. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. (2011) 15, 1239 / 1253
[2] Melican, K. et al. Bacterial infection-mediated mucosal signalling induces local renal ischemia as a defense against sepsis. Cell. Microbiol. (2008) 10, 1987 - 1998
[3] Campbell, E. L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity (2014) 40, 66 - 77
[4] Schaffer, K. & Taylor, C. T. The impact of hypoxia on bacterial infection. FEBS J. (2015) 282, 2260 - 2266
[5] Möllerherm, H. et al. Differentiation and Functionality of Bone Marrow-Derived Mast Cells Depend on Varying Physiologic Oxygen Conditions. Front. Immunol. (2017) 8
[6] Branitzki-Heinemann, K. et al. Formation of Neutrophil Extracellular Traps under Low Oxygen Levels. Front. Immunol. (2016) 7, 518
[7] Möllerherm, H. et al. Hypoxia Modulates the Response of Mast Cells to Staphylococcus aureus Infection. Front. Immunol. (2017) 8, 541
[8] von Köckritz-Blickwede, M. et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood (2008) 111, 3070 - 3080
[9] Chow, O. A. et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe (2010) 8, 445 - 454
[10] Zeitouni, N. E. et al. Measuring oxygen levels in Caco-2 cultures. Hypoxia (Auckland, N. Z.) (2015) 3, 53 - 66
[11] Zeitouni, N. E. et al. Hypoxia Decreases Invasin-Mediated Yersinnia enterocolitica Internalization into Caco-2 Cells. PLoS One (2016) 11, e0146103


