Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Perfusion Culture of Cell-seeded 3D Scaffolds for Tissue Engineering
In-line Oxygen Measurement in Perfusion Bioreactors
R. Santoro, B. Pippenger, I. Martin, and D. Wendt
Department of Surgery and Research, University Hospital, Basel, Switzerland
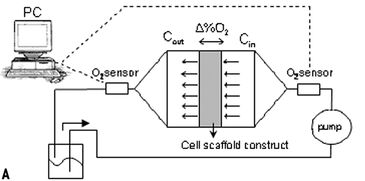
Since oxygen has a low solubility in culture medium it must be supplied to cells within 3D scaffolds via constant perfusion of the medium. The oxygen content of the medium before and after having passed by the cells can then help to determine whether the flow rate applied is sufficient. PreSens flow-through cells with integrated chemical optical sensors can be incorporated in the construct inlet and outlet and allow in-line measurement of oxygen tension. Normoxic oxygen supply creates homogeneous grafts with a uniform distribution of cells.
Bioreactors in Tissue Engineering
Tissue engineering, sitting at the crossroads of biology, chemistry and material sciences, has grown from conjecture into a "hot topic" field of research, with the final aim of generating human grafts applicable as autologous substitutes of injured tissues. Tissues are comprised of cells in a 3D conformation. Therefore, everything that happens to any given cell is not just a result of a 2D exchange, but a multitude of interactions, from biological to mechanical, all combining to result in a specific message to the cell.
To better reproduce and understand this complex system in vitro, perfusion bioreactors are used. In order to generate homogeneous tissue grafts, cells can be initially seeded into porous 3D scaffolds with a uniform distribution, thereby establishing a template for spatially uniform ECM deposition. The cells seeded within the interior regions must then be supplied with sufficient nutrients during prolonged 3D culture in order to maintain viability and support the production of ECM, possibly requiring the application of convective fluid flow.
Specific tissues have specific needs in regards to nutrient supply, mechanical stimulation and gas supply. Knowing what environmental conditions are being applied to the cultured scaffold are thus of critical importance.
Specific Bioreactor Systems
We previously described an integrated bioreactor system to allow prolonged perfusion of culture medium through 3D constructs following cell seeding by perfusion, within a single device. To continuously monitor the range of oxygen levels in the perfused constructs, in-line oxygen sensors were incorporated within the culturing pathway near the inlet and outlet of the constructs. According to the low frequency of our cell growth signal, we chose to measure the oxygen tension in the medium with the low sampling rate of one sample every 10 minutes.
Growing HAC Cells in 3D Scaffolds
Using the developed bioreactor system, we then tested the hypothesis that cells seeded uniformly throughout a 3D scaffold, when cultured under direct perfusion supplying a normoxic range of oxygen, will generate a homogeneous graft with a uniform distribution of cells and EMC. The hypothesis was tested using a human chondrocyte / foam scaffold model system. Adult human articular chondrocytes (HAC) were perfusion-seeded into 4.5 mm thick foam scaffolds at a rate of 1 mm/s.
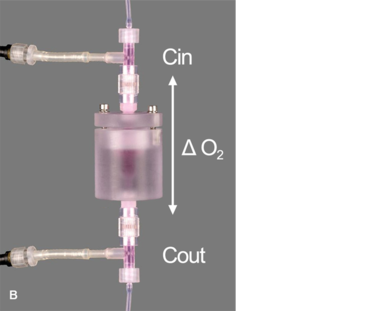
In order to eliminate artifacts (i.e. inadvertent media re-oxygenation), associated with sampling and off-line analysis, in-line oxygen sensors were required. However, a perfusion system for the current tissue engineering application imposes unique requirements for the sensors (e.g., small size, accurate at low flow rates, long-term stability), necessitating non-traditional sensor technologies. Therefore, flow-through chemical optical oxygen sensors by PreSens, based on the quenching of luminescence by oxygen, were incorporated into the culturing perfusion loop (Fig. 1, A + B). The sensors do not consume oxygen, are independent of the flow rate and maintain long-term stability.
A fiber optic cable transmitted the optical signal between the sensor and the Fibox 3 oxygen transmitter (PreSens GmbH, Germany). The transmitter was connected to a PC and via the software a constant sampling rate was set. Sensors were connected directly at the inlet and outlet of the scaffold chamber, in order to prevent oxygen diffusion into the system through tubing and other components, as seen in preliminary experiments, if sensors were placed further upstream / downstream. Nitrogen-infused water (i.e., 0 % oxygen) was perfused through the system to demonstrate that negligible oxygen from the incubator was diffusing into the bioreactor between the inlet and outlet sensor.
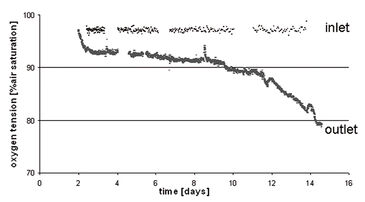
The test showed that at all times throughout the culture period, oxygen tensions measured at the inlet remained near saturation levels (i.e., 95 % air saturation, at equilibrium with the incubator atmosphere, set at 5 % CO2 - 19 % O2), and those measured at the outlet remained above 80 % air saturation (Fig. 2).
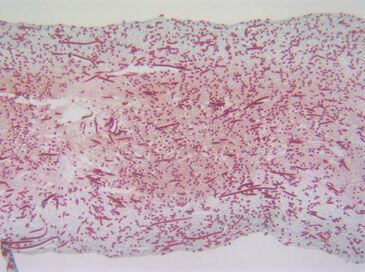
These data confirmed that at the flow rate used, the bioreactor maintained an efficient and rather consistent oxygen supply to the cells within the perfused constructs, close to normoxic levels. Successful long term cultivation of HAC cells was possible and microscopic data revealed that a homogeneous tissue formed (Fig. 3).
Conclusion
Maintaining a normoxic range of oxygen in the bioreactor system, it was possible to culture HAC for long term, and generate a homogeneous tissue with a uniform distribution of viable cells and cartilaginous extracellular matrix.
Observing the input signal it was possible to detect that at our sampling rate the sensors were not affected by the use, showing no drift even over long term measurement. The flow-through cells with integrated oxygen sensors were a useful tool to monitor the oxygen supply in perfusion culture. The optosensoric system by PreSens produced reliable results and might contribute to improving tissue engineering in the future.