Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Real Time Respiration Measurements
Measuring the aerobic respiration of complex microbial communities
B. R. Roller1, Z. M. Lee1, K. E. Studer-Rabeler2, and T. M. Schmidt1
1Dept. of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, USA
2Coy Laboratory Products, Grass Lake, MI, USA
Many methods for measuring aerobic respiration, including electrodes and colorimetric assays, limit the capacity for obtaining replicate measurements or the ability to detect oxygen consumption in real time. The OxoDish® - a microtiter plate with integrated oxygen sensors - and SDR SensorDish® Reader offer the potential for making twenty four simultaneous respiration measurements in real time, benefitting ecologists measuring the respiration of complex microbial communities.
Our aim was to optimize the SDR OxoDish® system for measuring the rate of oxygen (O2) consumption of pure cultures of bacteria and in soil slurries of complex microbial communities. Soil slurries are particularly challenging due to the potential for physical settling of soil particles on the optode. Two modifications of the OxoDish® were needed to measure O2 consumption accurately for these samples: minimizing O2 influx into the wells of the microtiter plate and maintaining a well-mixed solution so that O2 consumption was homogeneous throughout the depth of the well. A pure culture of Pseudomonas strain HF3 and forest soil were used to test modifications to the OxoDish®.
Materials & Methods
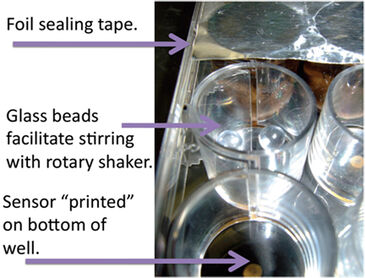
The influx of O2 into wells covered with adhesive foil was determined experimentally. A growth medium containing Resazurin, a colorimetric indicator of O2, and cysteine, a reducing agent that consumes O2, was added into 12 wells of an OxoDish® under anaerobic conditions in a vinyl anaerobic chamber / glove box. The wells were filled as close as possible to capacity and half of the wells were covered with adhesive foil and compared to uncovered wells in an ambient oxygen atmosphere at 25 °C and 100 RPM on a rotary shaker. The effect of glass beads, used to promote mixing beyond rotary shaking, on O2 consumption was tested with sterile growth medium as the negative control. Samples of Pseudomonas strain HF3 were removed from a balanced growth culture and placed into six OxoDish® wells, half of which contained 2 sterilized glass beads per well, and covered with adhesive foil (Fig. 1). O2 concentration was measured for each well every 15 seconds for 120-minute experiments at 25 °C and using rotary shaking at 100 RPM. In all rate measurements, the first 5 minutes of collected data were trimmed to permit time for equilibration. The bead and foil modifications to the OxoDish® were then utilized with soil slurry samples. Forest soil was sieved (2 mm), and resuspended 1 : 1 (w : v) in 10 mM MES buffer (pH 6) supplemented with 50 µg/mL cycloheximide and 2.3 mM sodium pyrophosphate. The soil slurry was stirred for 30 minutes, filtered through 8 layers of cheese cloth and strained through either a 100 µm or 40 µm cell strainer for different particle loading. The slurry was then added to wells with two sterile beads as described for pure culture experiments and sterile buffer was used as negative control.
Oxygen Influx
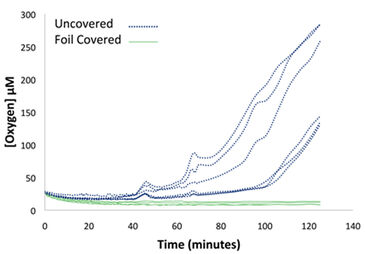
Wells that contained sterile anaerobic medium and were covered with adhesive foil showed no detectable O2 influx for more than 120 minutes, while uncovered wells began showing O2 influx after 40 minutes of exposure to an ambient atmosphere while shaking at 100 RPM (Fig. 2). The 40 minute delay in SDR detection of O2 influx of uncovered wells can be attributed to cysteine consuming any O2 that diffused into the media. Resazurin confirmed the SDR readings, with 6 uncovered wells turning pink within minutes of removal from the anaerobic environment, indicating O2 influx, while 5 of the 6 covered wells remained clear, indicating no O2 influx over many hours.
Pure Culture Oxygen Consumption
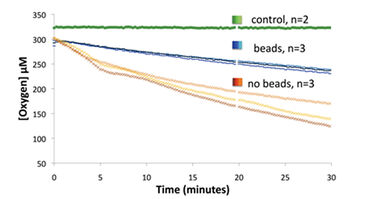
The second experiment measured the respiration of Pseudomonas strain HF3 and the effect of mixing with beads. Bacterial O2 consumption in wells that were mixed with beads was less variable than wells without beads, which is attributed to a more homogeneous solution throughout the well depth (Fig. 3). Using linear regression, the mean O2 consumption rate for samples with beads was 5.74 nmol/minute and replicates had a coefficient of variation (c. v.) of 6.7 %. The mean rate for samples without beads was 11.86 nmol/minute (c. v. = 18.9 %). The rates of O2 consumption have not been corrected for ingress of O2, but they are adequate for comparisons of variability between treatments. There was no detectable effect of cells settling on the optode when stirred with beads, as the rate of O2 consumption doubled after one doubling time of the pure culture population (data not shown). This indicates the solution was homogeneous and not experiencing a shift in growth when transferred from a flask to a sealed OxoDish® well. The specific O2 consumption rate with beads (mean 2.0 x 10-7), while not corrected for ingress, is similar to the rate measured by microrespirometry (mean 3.4 x 10-7).
Soil Slurry Oxygen Consumpiton
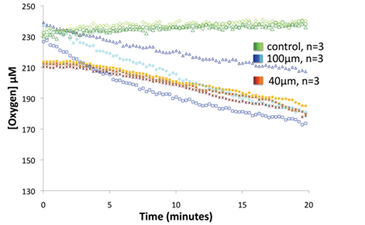
The beads and foil modifications to the OxoDish® were then extended for use with a complex microbial community. O2 consumption rates were measured for a soil slurry strained through two different sized cell strainers for different particle loading. The biggest difference between the two particle loading treatments came in the variability of measurement, with the 40 µm-strained samples having a coefficient of variation for the mean O2 consumption rate of 7.08 %, which is much smaller than the 100 µm-strained samples coefficient of variation of 35.05 % (Fig. 4). The decrease in variability for 40 µm-strained samples is attributed to increased homogenization of the soil slurry in the well, with the 100 µm-strained samples having too many particles that settled.
Conclusion
The SDR used with an OxoDish® is useful for the microbial ecology research community due to its capacity for replication and real time measurements. The OxoDish® can be modified with adhesive foil to minimize O2 influx into the wells of a microtiter plate and with glass beads to maintain homogeneous solutions throughout the depth of the well. Future investigations of complex microbial communities using the SDR and OxoDish® can measure O2 consumption while manipulating multiple variables simultaneously, where a large capacity for replication is necessary.