Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Simultaneous Monitoring of pH and Oxygenation in Microvolumes of Blood
Fiber optical pH microsensors and miniature broad range spectrophotometry advance blood function analysis
Michael Oellermann, Hans-Otto Pörtner, and Felix C. Mark
Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
The implementation of a pH microsensor and a miniature broad range spectrophotometer in a gas diffusion chamber facilitated the pH dependent analysis of blood oxygen binding properties. Parallel recordings of pH and pigment absorbance in only micro-volumes of unbuffered blood at freezing temperatures yielded highly resolved oxygen equilibrium curves.
For more than a century researchers have developed and refined devices to asses and comprehend the complex physiology of oxygen transport, leading to a variety of currently employed methods [1]. However, despite technological progress, methodological challenges are still to be met, particularly faced by biologists studying environmental effects on non-model organisms. Such challenges comprise:
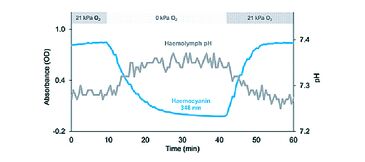
(1) The analysis of blood oxygen binding requires to control or monitor various factors such as temperature, carbon dioxide and particularly pH. pH however, varies in response to changing blood pigment oxygenation due to the uptake or release of protons by the pigment itself (Fig. 1). Added buffers stabilize pH during experiments but may mask or distort relevant responses of oxygen transport to such pH changes as they occur in vivo [2]. Comprehensive understanding of oxygen transport thus requires monitoring of blood pH simultaneous to pigment oxygenation in unbuffered blood [3].
(2) Frequently, sample volumes are limited to only a few microliters due to small organisms, problematic blood vessels or sub-sampling from live specimen. Yet, many devices consume large sample volumes (> 400 µL) for each measurement, also due to enhanced volume requirements by conventional pH electrodes, thus limiting the number of experimental replication or parameters measured.
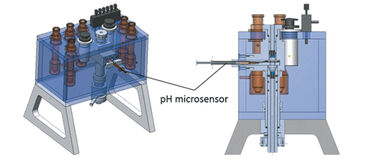
(3) At low experimental temperatures (< 5 °C) conventional pH electrodes perform poorly due to unstable and slowly responding signals, which hamper blood function analysis of e. g. polar organisms. To overcome these challenges we equipped a gas diffusion chamber (Eschweiler Co., Kiel, Germany) designed and described by Niesel and Thews [4], and Sick and Gersonde [5], with a PreSens pH microsensor and a miniature broad range spectrophotometer.
Materials & Methods
Technical Modifications
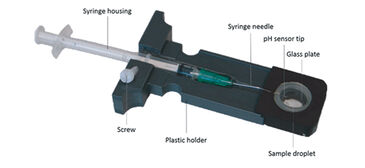
Gas diffusion chambers are commonly used to assess blood oxygen binding of minute blood samples by monitoring changes of absorbance at characteristic wavelengths in response to changing concentrations of oxygen, carbon dioxide and nitrogen gas. We modified a gas diffusion chamber: (1) by connecting a digital miniature broad range spectrophotometer and a Deuterium-Halogen (i. e. UV and visible light) light source (Ocean Optics, Germany) via fiber optic cables and collimating lenses directly above and below the blood sample to direct the light beam through the sample droplet; (2) and by implementing a pH microsensor (PreSens, Germany) as followed (Fig. 2): The syringe housing of the pH microsensor was fitted by milling a cylindrical channel into the sample glass holder and fixed via plastic screw (Fig. 3, patent number 10 2013 011 343 at DPMA). The steel needle covering the sensor was slightly bent (ca. 160° angle) to assure entry of the sensor tip into the droplet. The needle was then inserted through a silicone ring and the sensor tip moved into the edge of the sample droplet to avoid excessive bleaching by the light beam. The silicone ring allowed the pH microsensor to enter the gas tight chamber without causing gas leakage.
Blood function analysis
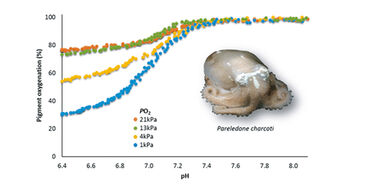
Blood function analysis was performed on cell free haemolymph from Antarctic octopods collected on the RV Polarstern cruise in March 2012. Prior to each measurement the pH microsensor was calibrated in six sea water standards (6.7 - 8.1) buffered with 40 mmol L-1 MOPS (3-(N-Morpholino)propanesulfonic acid) at 0 °C and the pH corrected to the Free Scale pH with Tris-buffered seawater standard (Dickson, CO2 QCLab, USA). Haemolymph was thawed on ice, mixed with 0.8 µL of 0.2 mmol L-1 NaOH (10 µmol L-1 final concentration) to raise pH above 8.0 and then 15 µL were spread on the glass plate. To calibrate oxygen equilibrium curves maximum absorbance was recorded at 100 % oxygen and minimum absorbance calculated by regression analysis [6]. Measurements were performed at constant oxygen partial pressure (pO2, 21, 13, 4, 1 kPa) and at continuously decreasing carbon dioxide partial pressure / pH (pCO2, 0 - 10 kPa / approx. pH 8.1 - 6.8).
Absorbance and pH Measurements of Unbuffered Octopus Blood
By means of the modified gas diffusion chamber we were able to analyze pH dependent oxygenation of Antarctic octopod haemocyanin in only microvolumes of unbuffered haemolymph (Fig. 4). The continuous and simultaneous recording of absorbance and pH at only moderate recording intervals (one measurement per 30 s) led to highly resolved oxygen equilibrium curves. The small probe diameter of 150 µm allowed complete submergence of the probe tip in the 15 µL sample droplet. Clogging of protein at the sensor tip and thus signal disturbance did not occur in octopod blood due to lacking haemostasis. Unlike pH electrodes pH microsensors provided stable and rapidly responding pH recordings at 0 °C experimental temperature (Fig. 4). Pigment oxygenation decreased in a sigmoidal manner towards lower pH, underlining the pH dependence of oxygenation typical for cephalopod haemocyanin [3]. Antarctic octopod blood showed a high oxygen affinity at 0 °C marked by incomplete unloading of oxygen at low pH and low oxygen partial pressures (Fig. 4).
Conclusion
The implementation of a PreSens pH microsensor and a miniature broad range spectrophotometer in a gas diffusion chamber overcame current methodological challenges in functional blood analysis particularly faced by experimental biologists who evaluate pH sensitivity of pigment oxygenation in minute volumes of unbuffered blood. Replicated measurements along pH gradients can be run at various oxygen partial pressures and temperatures, from specimens that supply only limited sample volumes without the need to pool samples. Simultaneous and continuous recordings of pH and absorbance significantly improved data resolution, allowing more detailed analysis of oxygen binding curves. Stable and rapidly responding pH signals at freezing temperatures facilitated the blood functional analysis of an Antarctic octopod, which showed a high oxygen affinity of its blood pigment haemocyanin. Future implementations may involve pO2 or pCO2 microsensors to perform conventional but finely resolved oxygen binding experiments along a pO2 gradient or to assess pH independent effects of CO2 binding to blood pigments.
References:
[1] Oellermann M., Pörter, H.-O. and Mark, F. C. (2014). J. Exp. Biol. 217, 1430 - 1436
[2] Brix, O., Colosimo, A. and Giardina, B. (1994). Mar. Freshw. Behav. Physiol. 25, 149 - 162
[3] Pörtner, H.-O. (1990). J. Exp. Biol. 150, 407
[4] Niesel, W. and Thews, G. (1961). Pflügers Archiv 273, 380 - 395
[5] Sick, H. and Gersonde, K. (1969). Anal. Biochem. 32, 362 - 376
[6] Oellermann, M., Pörter, H.-O. and Mark, F. C. (in prepatation). Blue blood on ice: Modulated blood oxygen transport facilitates cold adaptation and eurythermy in an Antarctic octopod