Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Online Measurement of Hypoxia in an Incubator
Hypoxia literally means 'little oxygen' and denotes an oxygen deficiency in body cells and tissues. In 2019, the Nobel Prize in Medicine was awarded to three hypoxia researchers. William Kealin Jr. (USA), Sir Peter Ratcliffe (UK) and Gregg Semenza (USA) have uncovered the molecular mechanisms of how cells measure and adjust oxygen availability - one of the most important processes in life. Thanks to this pioneering research, we know much more about how different levels of oxygenation regulate fundamental physiological processes.
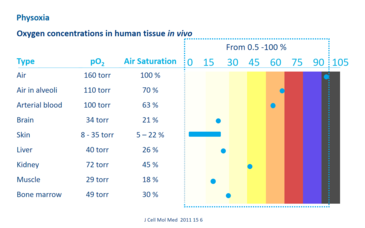
Cells measure their oxygen levels in some way and can then adjust their needs accordingly. In order to maintain normal cell functions, the oxygen content must be kept in a relatively narrow, physiological range that is individually adapted for each tissue, the so-called physoxic range. In vivo in the human body, the oxygen concentration varies significantly between different tissues, whereby the values are always much lower than the atmospheric oxygen content (Fig. 1). Physiological values that are well below the atmospheric oxygen content are usually referred to as hypoxia (= lack / undersupply of oxygen), although they actually correspond to the in situ normoxia.
The natural adaptation to hypoxically low oxygen concentrations is largely mediated by the transcriptional activation of genes, which enable short-term (e.g. glucose transport) and long-term (e.g. angiogenesis) adaptation mechanisms. This creates a balance between oxygen demand and oxygen supply. A key regulator of cellular responses is the transcription factor HIP-1 (hypoxia-inducible factor-1), which activates a wide range of genes that for example control energy metabolism, the growth of blood vessels or the synthesis of oxygen-transporting erythrocytes.
In most cell culture models in the laboratory, the experimental oxygen values are based on those provided in the standard cell culture incubators (without special regulation of the oxygen concentration). In humidified cell culture incubators, which are kept at 5 % CO2 as standard, in addition to the atmospheric gases, evaporated water molecules also generate a partial gas pressure, pH2O, of around 50 mbar (at 37 °C and 1013 mbar). Under these conditions, the oxygen content corresponds to 18.6 % O2 (v/v), which corresponds to an oxygen partial pressure (pO2) of 141 mmHg (Wenger et al. Hypoxia (Auckl). 2015 Sep 18; 3:35-43). In reality, no cell ever in the body has such a high oxygen concentration.
Experiments have already shown several times that cells cultivated in an oxygen-deficient culture (hypoxia) grow faster, live longer and show less stress, it is particularly important in laboratory experiments to optimize cell growth using an oxygen atmosphere that mimics the in vivo conditions as closely as possible.
Although the importance of hypoxia in biological processes is known, it is extremely difficult for most researchers to precisely control and monitor hypoxic conditions, as only sophisticated instruments can precisely control temperature, humidity and gases (CO2 and O2) in the incubator.
There are currently different ways of creating hypoxic conditions for cultured cells.
One of them involves the use of modular, airtight gas chambers in a standard CO2 incubator. By using such incubation chambers, which are placed in the standard incubator, the oxygen concentration is reduced in vitro in order to enable long-term cell growth under hypoxic conditions.
Another method that is often used is hypoxic cultivation in a so-called 'tri-gas' incubator, although this is a wrong description. Only two gases are added - carbon dioxide (as is usual) and nitrogen - to lower the oxygen content.
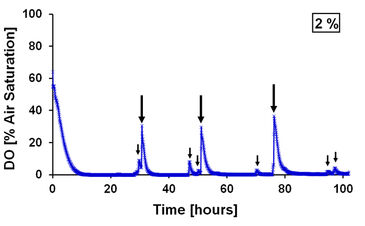
In both cultivation options, however, real-time visualization of hypoxia or cellular processes in response to hypoxic conditions becomes problematic if the cells are used for further analysis, e.g. using microscopic imaging, as they need to be removed from the incubator. When the incubator is opened, there is a renewed supply of oxygen from the ambient air, which can lead to misleading results because the oxygen concentration rises again within a very short time (Fig. 2). The time required to adjust the oxygen content in the medium, which was filled into the culture vessels under ambient air, to the atmosphere of the incubator is often underestimated, too.
In addition, some of the usual hypoxy detection markers such as pimonidazole or EF5 require fixation and thus killing of the cells with subsequent immunostaining. Even with fluorescent dyes that can be used in living cells, the problem of the ambient oxygen concentration arises. It is precisely for this reason that it is becoming more and more common to determine the actual oxygen concentration in the cultivation vessel itself using measuring devices.

The PreSens portfolio includes various suitable chemical-optical sensor systems to determine the current hypoxia status of a cell culture without oxygen consumption, non-invasively and without removing the culture vessels from the incubator.
For cell cultivation in multiwell plates, PreSens offers the SensorDish® Reader to determine the current hypoxia status. The SensorDish® Reader is a small 24-channel reader for recording oxygen or pH in multiwell plates (SensorDishes®). These contain a sensor spot at the bottom of each well that is read out non-invasively through the transparent bottom. SensorDishes® for oxygen (OxoDish®) and pH (HydroDish®) are available in 24-well and 6-well formats. The oxygen sensors on the bottom of the OxoDish® measure the oxygen content in the medium in their immediate vicinity. They can be used from an oxygen content of 1 % O2 in the incubator atmosphere.
The non-invasive PreSens VisiSens™ measuring principle enables imaging in static (not shaken) cell cultures in multiwell plates and T-flasks. It combines fluorescent sensor foils for oxygen, pH or CO2 with 2D reading technology to visualize the distribution of the respective parameter in the cell culture. The biocompatible sensor foils can be integrated into various transparent cultivation vessels. The cells are cultivated directly on the sensor foils, which means that the measurements are made directly in the pericellular microenvironment of the cells.
Both the SensorDish® Reader and the VisiSens™ detection unit can be installed directly in the incubator to ensure constant culture conditions (temperature, oxygen, CO2). Processes in cell cultures with an oxygen content of 1 % O2 or more in the incubator atmosphere can be monitored in real time.