Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Karin Benz and Jürgen Mollenhauer
Regenerative Medizin II, NMI Reutlingen, Markwiesenstraße 55, 72770 Reutlingen, Germany
Hydrogel-based 3D cell culture systems offer particular advantages for tissue-engineering in cell-based therapy for disc-degeneration. However, light microscopical control of both culture quality and morphological differentiation is not possible within this dense matrix. For a judgement of the quality, alternative parameters have to be considered. In the present study, availability of oxygen within culture medium and kinetics of the pH value are analyzed in this regard by using the SDR. Both parameters are key factors for a successful and reliable in vitro cultivation and differentiation.
Growth Control in 3D Culture
Autologous chondrocyte transplantation is a modern experimental therapy for treatment of degenerative disc diseases. Even repair of disc damages by means of grafted chondrocytes appears feasible in the near future. It must be considered, however, that under in vivo conditions the oxygen concentration within the disc nucleus is very low. Since chondrocytes are used to this situation, transfer into a normoxic culture environment could be harmful. Sensitive processes of in vitro cell proliferation and differentiation can only be achieved routinely by strict maintenance to defined culture parameters. Chondrocytes grow notably better within a gel matrix rather than in a monolayer culture because the spatial environment better resembles the situation within the disc (Fig. 1). Accurate measurements of oxygen consumption during cultivation allow conclusions on the physiological state and quality of culture conditions. Both culture parameters were so far not accessible for continuous monitoring - mainly due to lack of the required miniaturized technique. The optosensoric measuring system SDR of PreSens now enables non-invasive detection of both culture parameters close to the cells.
Non-invasive Online Detection
Characterization of kinetics of dissolved oxygen and pH value was performed with a well-established 3D culture method. Three defined cell densities of 0.1, 0.5 and 1 x 106 cells/ml were cultured on a proprietary hydrogel matrix for about two weeks in OxoDish® and HydroDish®, respectively. Each well contained 2 ml of buffered culture medium of pH 7.4. During the study, the medium was changed three times - on days 2, 5 and 8 - in the samples with cells as well as in the controls. Online monitoring started shortly after seeding the cells. Optical controls were performed on days 6 and 9. For this purpose, the multidishes were shortly removed from the standard incubator atmosphere of 5 % CO2 / 95 % relative humidity. Oxygen kinetics were obtained calculating the average of three replicates per cell density. Cell-free cultures served as control.
Monitoring of Oxygen and pH Kinetics
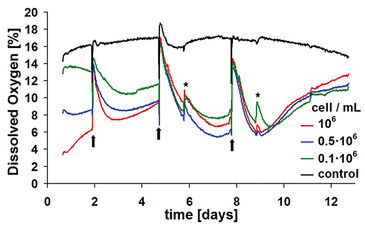
Samples with highest density reveal the greatest oxygen consumption and, accordingly, the lowest content in dissolved oxygen (DO) within the culture medium (Fig. 2). At medium exchange, DO increases again to a value similar to the starting level of 14 to 16 % oxygen, according to the preceding equilibration of the medium. Depending on the metabolic activity of the sample, the oxygen content decreases again within short time to a new minimum. After reaching a minimum point, DO rises again within all samples. The reason for this, presumably, is a strong reduction of metabolic activity since chondrocytes use the Crabtree-effect. The oxygen kinetic is determined by further complex factors: One factor accounting to this effect is cell proliferation. Within the sample with 0.5 x 106 cell / ml, already after the second medium change a more pronounced decrease in oxygen kinetics is evident compared to the highest initial cell density, indicating a higher metabolic activity. Further increase of cell densities, therefore, results in diminished metabolic activity.
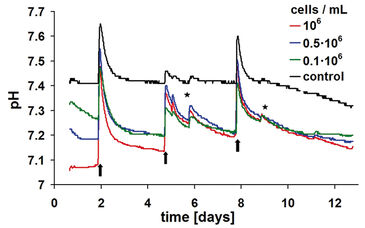
Like the oxygen measurements, the pH kinetics reveal characteristic dynamics according to the length of cultivation and the initial cell density as well (Fig. 3). Apart from changes of culture medium and effects due to openings of the incubator door, the controls display a largely stable pH value. The pH values of the samples with cells decrease earlier, however, without falling beyond values of pH 7.0 (cf. Crabtree effect, above). At the higher initial cell densities of 1.0 x 106 and 0.5 x 106 cells / ml it takes only about one day in order to reach the respective pH minimum.
Due to the change of culture medium, a brief pH shift beyond the physiologically optimal initial pH value takes place for the samples with cells as well as for the cell-free controls. Media that have not been pre-incubated in CO2 atmosphere reveal, as a consequence, enhanced pH values until they are equilibrated again with the incubator atmosphere. This equilibration can last up to one day because gas exchange happens only via passive diffusion. A similar effect is detected also at opening of the incubator door: Even at short opening, CO2 leaks out of the incubator atmosphere almost completely. Consequently, the pH value of the culture medium increases. The superimposing effects of the steep rise and fall of pH after change of culture medium and the metabolically determined downward trend impede an exact interpretation of the observed pH kinetics. In analogy to the oxygen measurement, a less pronounced downward trend points to a diminished metabolism caused by lack of substrate. By means of continuous pH detection, however, it can be avoided that the pH decreases to adverse low values caused by an enhanced metabolic activity. The latter could inhibit cellular activity or even cause cell damage. Online monitoring of pH thus provides valuable hints on the time for necessary changes of culture medium.
Culture Control
Non-invasive monitoring of DO and pH with the SDR is well suited for in-process assessments of culture quality and determination of the optimum time for medium change in 3D cell cultures. It is likely that using the culture system described here enhances the quality of autologous chondrocytes for transplantation. Through DO kinetics, standard protocols for an even more efficient cultivation of chondrocytes may be derived, and the optimum time for chondrocyte harvest determined. Furthermore, the SDR enables for the first time a precise insight into 3D cell cultures by detecting available oxygen. Precise measurements and the advantage of continuous data acquisition underline the high potential and advantage for future applications of this system in tissue engineering. Besides monitoring metabolic substrate acidification, the pH measuring system is also an exceptional instrument for protection and control of buffer parameters in a given culture medium. Using such instrumentation, companies can enhance the quality of cell and tissue products and sooner realize their therapeutic benefit to patients.
Application note adapted from
BioProcess International, 12/2008, 54 - 59.