Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Diffusive / Dispersive Reaction Fronts in Groundwater
2D visualization of oxygen and pH using the VisiSens imaging system to study Fe2+ oxidation under flow-through conditions
Christina Haberer1, Muhammad Muniruzzaman1, Massimo Rolle1,2, and Peter Grathwohl1
1Department of Geosciences, University of Tübingen, Germany
2Department of Civil and Environmental Engineering, Stanford University, USA
Quasi 2D flow-through experiments were performed in heterogeneous porous media to study the oxidation of ferrous iron in groundwater using the VisiSens™ imaging system for oxygen and pH. The supply of oxygen occured by mass transfer from the atmosphere to anoxic groundwater and was additionally affected by the reaction with ferrous iron. We observed that the ferrous iron was preferably oxidized within a coarse-material inclusion, acting as a high-permeability zone that caused flow focusing and favored diffusive / dispersive mixing of reactants. pH was found to be lower in this zone because the oxidation of ferrous iron results in the formation of protons. Due to the interaction between iron minerals and xenobiotic and geogenic pollutants, the outcomes of our investigation are relevant for a number of water quality issues.
Diffusion and dispersion are key mechanisms for mass transfer of volatile compounds to or from groundwater systems. The transfer of oxygen to groundwater plays an important role for many biogeochemical reactive processes. Particularly relevant are the reactions with iron, which is a common constituent in soils and groundwater. Changes in redox and pH conditions influence iron speciation and reaction kinetics. The reaction between dissolved oxygen and ferrous iron (Fe2+) leads to the formation of ferric oxy-hydroxides and results in the propagation of reactive fronts in the subsurface. This plays a significant role in determining the environmental fate of many organic and inorganic pollutants interacting with iron minerals, such as the migration of arsenic in groundwater. Understanding the dynamics of iron oxidation fronts is, thus, of critical importance to quantitatively describe and predict arsenic transport. We performed quasi 2D flow-through laboratory experiments to study mass transfer of oxygen in heterogeneous porous media and its influence and control on iron oxidation. The VisiSens™ A1 and A2 systems were applied to map the spatial and temporal 2D distribution of oxygen and pH, which are key parameters to capture the dynamics of iron oxidation.
Materials & Methods
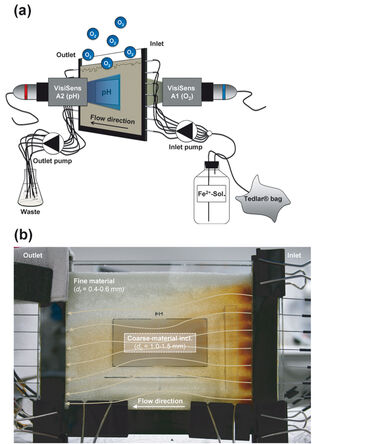
Figure 1a shows the experimental set-up. The flow-through chamber had inner dimensions of 17 x 12.5 x 0.5 cm (L x H x W) and was filled with glass beads as porous medium. A coarse-material inclusion (grain diameter: 1.0 - 1.5 mm) was embedded within a finer matrix (grain diameter: 0.4 - 0.6 mm), which resulted in the convergence of flow in the high-permeability zone (Fig. 1b). We pierced hollow needles (outer diameter: 1.2 mm), connected to Fluran-HCA pump tubing, into the chamber. Via these connections and using a high-precision peristaltic pump (IPC 24, ISMATEC, Switzerland) aqueous solutions could be injected into and extracted from the chamber. We established horizontal flow (seepage velocity of 5 m d-1) by first flushing the flow-through chamber with an anoxic aqueous solution until steady state was reached. Afterwards, an oxygen-free Fe2+-solution (10 mg L-1 Fe2+, added as FeCl2 x 4 H2O; with pH 7) was injected. PIPES (1 mM, CAS: 5625-37-6) was added to both solutions to limit the maximum decrease in pH due to the reaction, and ionic strength was adjusted to 100 mM using NaCl. Mass transfer of oxygen occurred from the atmosphere across the unsaturated / saturated interface, into the anoxic groundwater where it reacted with ferrous irons to form ferric hydroxide precipitates:
4 FeCl2 x 4 H2O + O2 -> 4 Fe(OH)3 + 6 H2O + 8 H+ + 8 Cl-
To map the 2D distributions of oxygen and pH during the experiment, planar optrode foils (8 cm x 4 cm each; oxygen SF-RPSu4, pH SF-HP5R) were glued onto the inner sides of the flow-through chamber on the two opposite glass panes (Fig. 1a). The measurement signal was detected using the appropriate VisiSens™ cameras (detector units DU01 and DU02, respectively). A tube was mounted on each VisiSens™ camera allowing to depict an area of 1.9 cm x 2.4 cm when taking a picture. By taking several continuous pictures at different points in time the whole optrode foil was covered. We performed the flow-through experiments at constant room temperature (25 °C).
Results
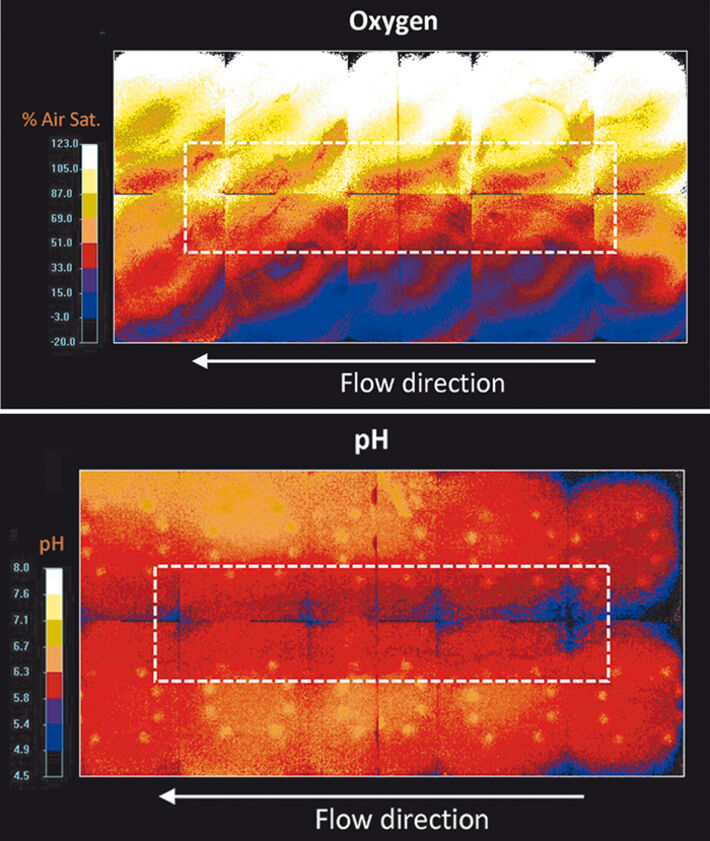
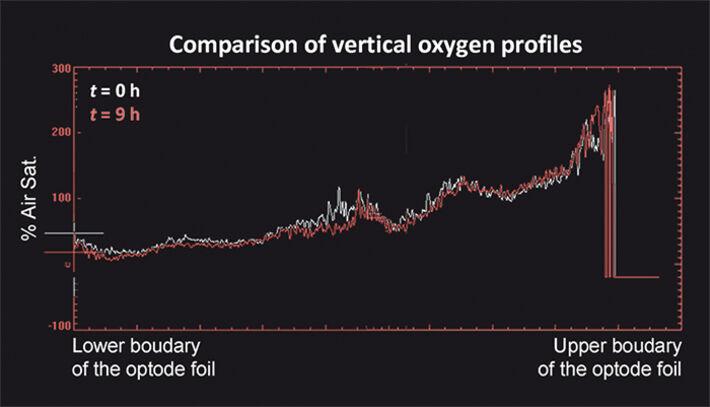
In the experiments oxygen was transferred from the atmosphere to the anoxic water. The presence of the high-permeability inclusion had a significant effect on the flow field and on the distribution of oxygen in the transition region between the unsaturated and the saturated zone. Due to the convergent flow field, water with high oxygen concentrations from above and very low concentrations from below was focused in the coarse-material inclusion. Fig. 2 (top) shows the oxygen distribution 9 hours after starting the injection of the Fe2+-solution into the flow-through chamber. The flow field and the distribution of oxygen also determined the region in which ferrous iron was oxidized and precipitated as ferric iron minerals. The presence of ferric hydroxide minerals could be clearly observed due to their dark orange color (Fig. 1b). Additionally, the spatial distribution of pH confirmed the presence of the reactive front in the high-permeability zone. In fact, the anoxic Fe2+-solution effectively mixed with the oxygen supplied across the capillary fringe and the oxidation reaction not only resulted in the precipitation of iron hydroxide but also caused a slight decrease of pH within the coarse-material inclusion (Fig. 2, bottom). This is likely to be caused by the newly formed iron hydroxide minerals which can catalyze the abiotic oxidation of Fe2+, thus, resulting in additional release of hydrogen ions.
Conclusion
In our experiments the VisiSens™ imaging systems were applied to map the 2D distribution of oxygen and pH on opposite sides of a quasi two-dimensional flow-through chamber. We were able to follow the spatial and temporal dynamics of these water quality parameters in heterogeneous porous media. In fact, applying the VisiSens™ A1 and A2 imaging systems allowed us to visualize the impact of porous medium heterogeneity on oxygen transfer. Furthermore, we were able to identify the high-permeability zone as the region in which oxidation of ferrous iron preferentially occurred. The insights gained in this study are relevant to improve the understanding of the propagation of reactive fronts in groundwater involving iron species, which are strongly coupled with the release and transport mechanisms of contaminants. VisiSens™ could be applied in many other investigations on this subject.