Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Effect of Oxygen Depletion on GDH Activity
Oxygen Monitoring in Shaken Cultures of C. glutamicum and C. diphtheriae
M. Böhme and A. Burkovski
Department of Microbiology, FAU Erlangen-Nuremberg, Germany
The impact of oxygen depletion in cultures of Corynebacterium glutamicum and two different strains of Corynebacterium diphtheriae was investigated under different cultivation conditions. Experiments were conducted to characterize the impact of low oxygen concentrations on a specific enzyme of the nitrogen metabolism - the ammonium transferring enzyme glutamate dehydrogenase (GDH). Oxgyen monitoring was performed using the oxygen meter Fibox 3 by PreSens. Growth rates in all tested Corynebacterium cultures were dependent on oxygen supply and increased the more oxygen was available. The investigated activity of GDH seemed not to be affected by low oxygen levels in C. glutamicum. In cultures of C. diphtheriae the GDH activity increased under certain conditions, but only very low activity values could be determined that might not be significant.
The assimilation of ammonium, which is known as the preferred nitrogen source of C. glutamicum, is mainly accomplished by NADPH-dependent glutamate dehydrogenase (GDH). The enzyme turns ammonium to L-glutamate, which makes GDH a biotechnologically important enzyme, as glutamate is needed in large amounts as a flavor enhancer by the food industry. Therefore one aim is to improve the efficiency of GDH. In aerobic bacteria like C. glutamicum and C. diphtheriae oxygen limitation can affect the metabolism and might also impair GDH activity. In previous studies by T. Müller [2] a decreased transcription of gdh in C. glutamicum under oxygen exclusion could be observed. The following experiments were conducted to further investigate the impact of oxygen depletion on growth rate, GDH activity and gdh transcription in cultures of C. glutamicum and C. diphtheriae. Different culture conditions with varying culture volumes and shaking speeds were tested. The Fibox 3 together with chemical optical oxygen sensors was used for non-invasive oxygen monitoring in the cultures over the complete cultivation period.
Materials & Methods
300 mL baffled glass shake flasks were used for the experiments, some of which had an oxygen sensors spot (PreSens) attached to the inside bottom wall. The coaster CFG, an adapter, was placed under the shake flask right opposite the sensor spot and connected to the oxygen meter Fibox 3 by PreSens to read the sensor response. The oxygen concentration was monitored and recorded throughout cultivation at a sampling rate of 30 seconds. A wild type strain of C. glutamicum ATCC13032 and two wild type strains of C. diphtheriae - DSM44123 and DSM43988 - were used for this study [1]. To examine differences in oxygen depletion under different culture conditions volumes of 25 mL, 50 mL and 100 mL at a shaking speed of 125 rpm and two additional cultures of 50 mL volume at shaking speeds of 100 rpm and 150 rpm were tested. For every series of tests four parallel cultures were inoculated in the shake flasks. In each series three samples of 15 mL were taken, each from a separate culture vessel, for glutamate dehydrogenase activity measurement and RNA hybridization: after 0.5 hours, 2.5 hours and 6.5 hours. The remaining shake flask was equipped with the sensor spot for oxygen monitoring. The gdh DNA fragment was amplified by colony PCR and subsequently purified by gel electrophoresis and extracted with NucleoSpin® Extract Kit (Macherey-Nagel, Düren). GDH activity was measured as described by T. Müller [2] and calculated with the following equation:
activity = (ΔA x V) / (ε x t x m x d)
Delta A is the absorbance decrease and V the total reaction volume. Epsilon is the extinction coefficient, t the reaction time, m protein in the reaction mixture (in mg) and d the layer thickness. Protein in the reaction mixture was measured with Roti-Quant® at oD595. Isolation of total RNA was performed with the NucleoSpin® RNA II-Kit (Macherey- Nagel, Düren). Sythesis of digoxigenin-labeled RNA probes and RNA hybridization was done as described by E. Hänssler [3].
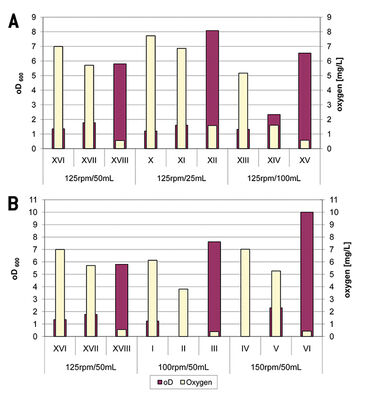
Oxygen Depletion & Growth Rate
Oxygen levels at 0.5, 2.5, and 6.5 hours of cultivation were compared with optical density at that time (Fig.1). It could be observed that the growth rate is dependent from oxygen supply both in C. glutamicum and C. diphtheriae. Varying conditions like different shaking speed and culture volume caused variations in oxygen supply. In sum the growth rate increased the more oxygen was available, matching already known data. When culturing fast growing bacteria like C. glutamicum it is very important to monitor growth rate and oxygen concentration in correlation. Because, for example, cultures of C. glutamicum shaken with 150 rpm showed a lower oxygen level but higher OD than cultures shaken with 125 rpm, which might have caused oxygen depletion over time. The oxygen consumption of slow growing bacteria like C. diphtheriae is really low, so it is nearly in balance with the oxygen input. In all series of experiments oxygen consumption had exceeded the oxygen input and the concentration decreased. No series reached complete oxygen limitation.
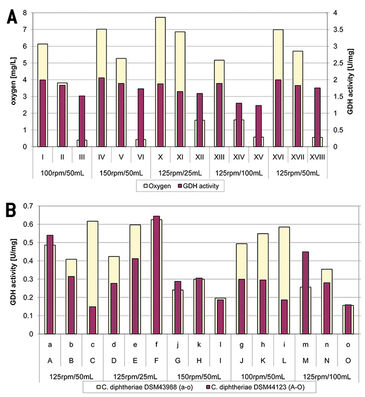
Impact of Oxygen Depletion on GDH
Comparing GDH activity and oxygen depletion for C. glutamicum revealed no strong correlation (Fig. 2A); a decrease in oxygen led to both strong and weak decrease in GDH activity. Moreover no significant correlation between a decrease in oxygen and the amount of gdh transcript could be observed. The decrease of GDH activity was in a range of 0.25 - 0.7 U/mg over 6.5 hours. These values are very low and might have been caused by a fluctuation or the result of other regulating processes. Nevertheless an impact of oxygen depletion on GDH activity cannot be excluded. In contrast to C. glutamicum a slight increase in GDH activity under some culture conditions could be observed in both strains of C. diphtheriae (Fig. 2B). But the measured values were really low and might not be significant. The oxygen concentration was more or less decreasing during cultivation in both strains of C. diphtheriae while GDH activity both increased and decreased. The dot blot for C. diphtheriae DSM44123 showed an increase in transcript (except: 25 mL / 125 rpm) which does not correlate with GDH activity measurement. This might indicate that glutamate dehydrogenase is regulated post-transcriptional. The dot blot and GDH activity measurement in the strain C. diphtheriae DSM43988 matched more or less but did not match with the results obtained from C. diphtheriae DSM 44123. As the results of the two strains differ, no clear conclusion can be given on the impact of oxygen depletion on GDH in C. diphtheriae.
Conclusion & Perspective
No certain impact of oxygen concentration on GDH activity could be observed in cultures of C. glutamicum and C. diphtheriae at the tested incubation conditions. As only very low GDH activity values could be measured, further tests using larger amounts of protein would have to be conducted. The results for C. glutamicum culture did not confirm the expected influence seen in the study of T. Müller [2]. Other conditions might have to be tested like cultivation at even lower shaking rates or without shaking. As a decrease in oxygen is expected to reduce GDH activity the reverse approach - additional oxygen input - could allow more detailed insight. These new approaches might give better or more significant results for GDH activity measurement.
Application note adapted from:
[1] Böhme, M. (2008), Oxygen depletion in cultures of Corynebacterium glutamicum and Corynebacterium diphtheriae and its effect on glutamate dehydrogenase, Bachelor thesis, FAU Erlangen-Nuremberg, Germany
References:
[2] Müller, T. (2005), Regulation of glutamate dehydrogenase in Corynebacterium glutamicum and its impact on nitrogen control. PhD Thesis, University of Cologne, Germany
[3] Hänssler, E. (2008), Regulation of glutamate dehydrogenase in Corynebacterium glutamicum, PhD thesis, FAU Erlangen-Nuremberg, Germany