Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
High Throughput Respiration Assay for Cytotoxicity Determination
A Method for the Evaluation of New Active Pharmaceutical Ingredients Using the SDR SensorDish® Reader
Marie-Theres Weil, and Tobias Werner
Institute of Analytical Chemistry, University of Applied Sciences Mannheim, Germany
The objective of this project was to develop a cytotoxicity assay with high throughput by monitoring the cellular respiration in cell culture. Cells were cultured in 24-well plates with integrated chemical optical sensors (OxoDishes®) and read out online and non-invasively with the SDR SensorDish® Reader. In consequence, the effect of different concentrations of cytotoxic and cytostatic substances on cell respiration could be easily determined. Results showed that an explicit distinction between cytotoxic and cytostatic compounds can be made by online monitoring of cellular respiration.
With an increasing number of new active pharmaceutical ingredients, it becomes more and more important to find efficient and fast screening approaches for industrial application. In this study a cytotoxicity assay with high throughput by monitoring cellular respiration in cell culture was developed. When cells are treated with cytotoxic compounds, they tend to become apoptotic and do not consume oxygen anymore. If the amount of oxygen diffusion into the medium is greater than the oxygen consumption of the remaining cells that survived, the overall dissolved oxygen content in the medium increases. In contrast to this, when treating cells with cytostatic compounds, the oxygen consumption should stay relatively constant as a result of stopping the proliferating process. Chinese hamster ovary cells were cultured in OxoDishes® by PreSens. The optical oxygen sensors located at the bottom of each well are read out online and non-invasively with the SDR SensorDish® Reader, which allows monitoring the oxygen uptake in all 24 wells of a plate simultaneously. Apart from other tests - not shown here - the effect of different concentrations of cytotoxic and cytostatic substances on cell respiration was determined using this system.
Materials & Methods
Cells were cultured in 24-well OxoDishes® and the chemical optical sensors at the bottom of each well read out with the SDR by PreSens. The SDR can be placed inside the incubator and allows online monitoring of oxygen in the cultures throughout the period of treatment. For cellular respiration experiments the Chinese hamster ovary cell line CHO-K1 was used. These cells were maintained in Ham´s F-12 medium supplemented with 10 % FCS and 1 % penicillin-streptomycin and incubated at 37 °C in a 5 % CO2 atmosphere. The optimal cell density was found to be 300.000 cells per well. This optimal density has to be determined for each cell batch, as cell growth changes with the number of cell passages. Cells were grown in 1 mL of medium for 24 hours. 1 mL of fresh medium was added containing the cytotoxic or cytostatic substances in different concentrations. The dissolved oxygen content was monitored every 10 minutes for 24 hours. After the respiration measurements, cell viability was directly assessed using the standard MTT test. All stock solutions of the test compounds were dissolved in Ham´s F-12 medium, if necessary 1 % final concentration of DMSO was used, and then diluted appropriately with medium to yield the desired concentrations. In order to determine the median lethal concentration LC50 of a cytotoxic compound, the dissolved oxygen content after 24 hours of treatment was plotted over the appropriate compound concentration. The resulting curve was fitted with a four-parameter sigmoidal concentration-response curve using OriginPro version 8.0.
Effect of Cytotoxic Compounds on Cellular Respiration
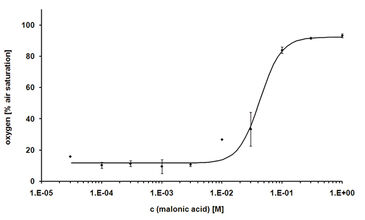
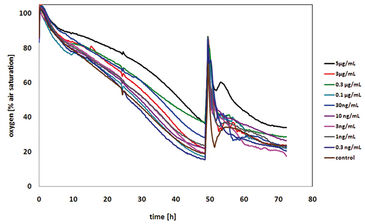
The first cytotoxicity tests were performed with malonic acid. Sub lethal concentrations of malonate did not affect the oxygen consumption of the CHO-K1 cells, which led to decreased dissolved oxygen content in the medium. As a result of high malonate concentrations, cell death was induced and thus, no more oxygen is consumed. Due to oxygen diffusion from ambient air, the dissolved oxygen increased up to 100 % air saturation (Fig. 2). The dissolved oxygen content after 24 hours of treatment was plotted over the corresponding concentration of malonic acid to determine the LC50 that was found to be 34 mM (Fig. 3). This value is in very good accordance to the LC50 value determined by the MTT test (31 mM). The literature value (4 mM) was a little lower, which is due to use of a different cell line. Further tests with other cytotoxic compounds were performed (results not shown, please refer to [1]).
Effect of Cytostatic Compounds on Cellular Respiration
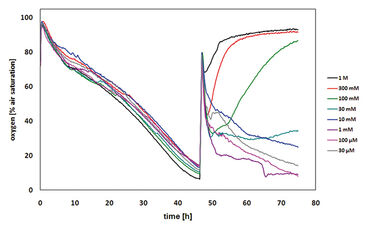
The same experimental set-up had been used for the assays with colcemid (demecolcin). Colcemid can depolymerize microtubules by limiting microtubule formation through inhibition of the spindle fiber formation and thus, arresting cells in the metaphase. The results gained from these assays proved that the cells treated with colcemid did not die. The oxygen content in the medium was relatively constant indicating a constant cellular respiration. The dissolved oxygen content before and after the treatment is about the same and thus, the cells constantly consume the same amount of oxygen per time period as the cell cycle is inhibited and no cell division is possible (Fig. 4). Furthermore, non-significant difference in oxygen consumption after 24 hours of treatment with different colcemid concentrations could be found. The variation of the dissolved oxygen content in the medium can be explained by the slightly different cell numbers in the consecutive wells. Thus, it could be shown that colcemid is not toxic to CHO-K1 cells, but rather a cytostatic agent. This result is supported by the MTT assay that confirmed the cell viability after 24 hours of treatment as none of the samples were found to be non-viable, and the different absorbance values might be explained by different cell numbers.
Conclusion
The results show that an explicit distinction between cytotoxic and cytostatic compounds can be made by online monitoring of cellular respiration. The results obtained with the SDR correlate with the MTT assay and therefore are a good indicator for cytotoxicity. Literature values for cytotoxicity compounds are about 10 times lower than the LC50 determined in the experiments shown here. Due to the vast differences between cell lines and primary cells, it is only possible to draw general comparisons. In summary the experiments proved that the SDR reader system provides a fast and easy-to-use online method to determine cytotoxicity of new pharmaceutical compounds.
Application Note adapted from
[1] M.-T. Weil and T. Werner, Cytotoxicity Determination: A method for the evaluation of new active pharmaceutical ingredients, GIT Laboratory Journal 2012 5 - 6