Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Improving Culture Conditions of Antibody-Producing Yeast
Use of Deep Well SensorDishes® for pH and DO Monitoring

Lotta Kirjavainen and Alexander Frey
Aalto University, Finland
Reliable screening set-ups are the key to rapid identification of genetic modifications or changes of cultivation conditions improving strain performance. Moreover, online monitoring of culture conditions and cell physiology would be desirable already in early phases of the screening process in order to obtain additional information. We have used SensorDishes® to optimize culture conditions for an antibody-producing Saccharomyces cerevisiae strain. Based on measurements of pH and dissolved oxygen (DO) concentration, we could show that increasing the buffering capacity of standard synthetic yeast media affects stability of the secreted antibody. Furthermore, we detected that the media modifications also affected dissolved oxygen levels during batch growth of the strain.
We have successfully engineered Saccharomyces cerevisiae strains that are capable of producing full-length antibodies (IgG) and glycosylating them with human type N-glycans [1, 2]. Currently, compared to other established cellular production systems such as CHO cells, IgG productivity of these engineered yeast strains is lagging behind and the production titers are far from being commercially competitive. In order to improve productivity, we regularly screen different types of deletion strain or DNA libraries for identification of genetic determinants improving IgG productivity [3, 4]. These libraries can include limited sets of candidate genes targeting a specific cellular process, for example protein folding, or genome-wide deletion libraries. The number of tested strains can include several hundreds. In our standard screening set-up using 96- or 24-deep well plates we are only assaying IgG titers and optical cell densities after 24 hours of growth.
In order to further improve the screening process, it would be desirable to obtain more insights into the cellular physiology and culture conditions. In particular, the behavior of the pH is of great interest, as we know from earlier experiments that the secreted IgG is most stable at neutral pH, whereas yeast grows preferably at acidic pH. A further complication is that the most commonly used yeast media are typically formulated using Yeast Nitrogen base (YNB), which contains the nitrogen source, salts, trace elements and vitamins but is only lightly buffered. Thus, over the time of a cultivation, the culture pH can drop quite strongly. Therefore, we tested whether an increased buffering capacity of the media affects IgG production of S. cerevisiae.
Materials & Methods
The SDR SensorDish® Reader was used for online monitoring of pH and oxygen in yeast culture inside Deep Well Oxo- and HydroDishes® (OD24-DW, HD24-DW, PreSens) equipped with covers (Applikon) ensuring homogeneous oxygen supply to all the wells. An optical shielding mask (SDR-OSM24, PreSens) was placed between the reader and the SensorDishes® to avoid that media fluorescence might interfere with the optical measurements. The system was mounted in a temperature controlled shaker using the System Duetz clamp system. The Saccharomyces cerevisiae strain YEK18, a derivative of the wildtype yeast W303α (leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) strain was used in the experiments. The strain contains a single copy of the genes encoding the light and heavy chain of a human IgG integrated into the HIS3 locus. Expression of light and heavy chain is under control of a galactose inducible promoter.
Colonies were picked from plates and inoculated into 3 mL of complete synthetic media (6.7 g/L YNB lacking amino acids, 2.0 g/L amino acid mix, 20 g/L raffinose) and grown overnight at 30 °C and 220 rpm. For the expression tests, OD24-DW and HD24-DW plates were filled with 3 mL of complete synthetic media supplemented with 50 µg/mL BSA and inoculated to a starting OD600 of 0.2. For testing of the pH effect, the media was supplemented with 0 to 50 mM of phosphate buffer, pH 6.5. The inoculated deep well plates were incubated at 30 °C and 250 rpm for 6 hours and IgG expression was induced with addition of 0.5 % galactose. The induced cultures were incubated at 30 °C for 18 hours at 250 rpm. During the whole cultivation time, dissolved oxygen concentration and culture pH were recorded every ten minutes.
Final OD600 was measured at the end of the cultivation. Samples for IgG determination were collected 18 hours after induction. Cleared culture supernatants were adjusted to 1 x PBT (PBS: 135 mM NaCl, 2.5 mM KCl, 10 mM Na2HPO4, 1.75 mM KH2PO4, containing 0.5 % Tween-20) and stored at - 20 °C until analysis. An enzyme-linked immunoassay (ELISA) run on a Hamilton Star Line liquid handling station was used to determine antibody concentration in the culture supernatants. Final raffinose and galactose concentrations were measured from cleared culture supernatants using HPLC.
Results
During batch growth of S. cerevisiae in synthetic media, the pH can drop to values as low as 4 within 24 hours of cultivation due to the low buffering capacity of the media. Under these acidic conditions, the stability of antibodies can be severely reduced leading to more extensive proteolytic degradation of the secreted molecules and ultimately to the underestimation of the amount of produced antibodies. In order to get additional insight into our currently used screening process we have used Deep Well Oxo- and HydroDishes®.
When growing the antibody producing yeast in the synthetic media, the initial pH value of around 5.7 to 5.8 dropped within the first five hours to a value below 5. Antibody titers after 24 hours were very modest and additional experiments showed that the peak antibody titers were reached already after 12 to 16 hours and after this time point, a rapid decline in the titer of secreted antibody can be observed. Therefore, we investigated whether the addition of buffering substances could overcome this shortcoming.
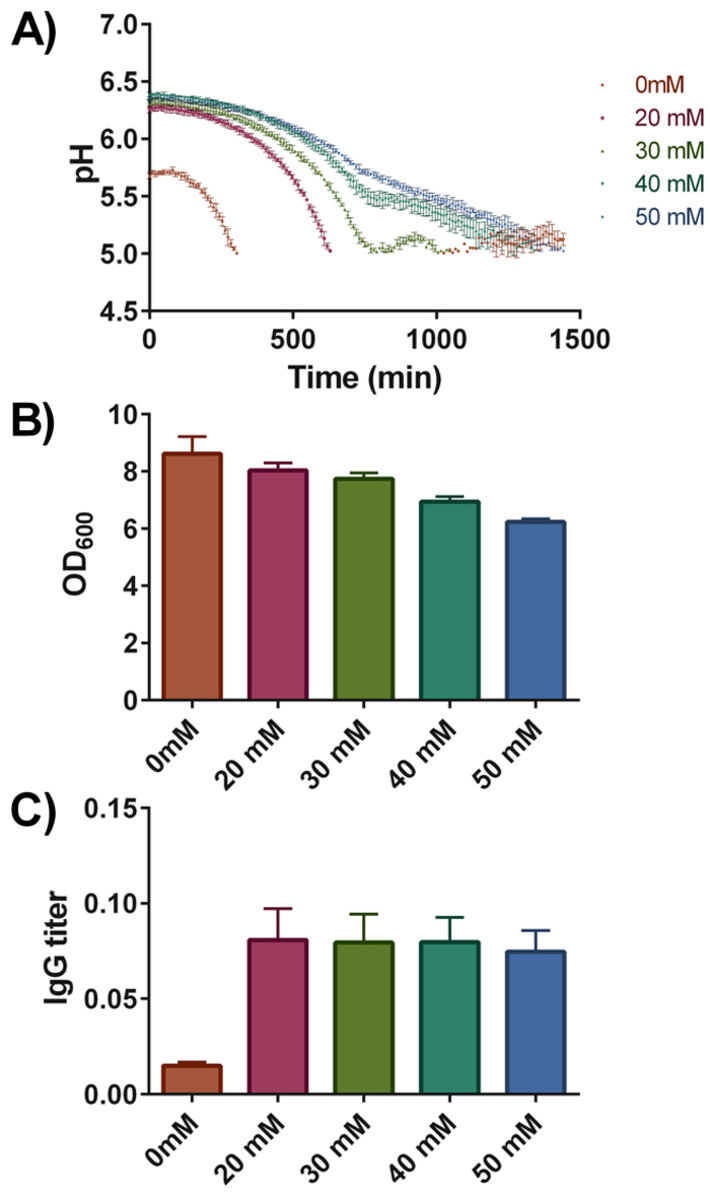
We have grown YEK18 in synthetic media supplemented with increasing amounts of phosphate buffer (0 to 50 mM). Pre-cultures were grown overnight and three replicate cultures were inoculated to a starting OD600 of 0.2 into the wells of the Deep Well HydroDishes® that were positioned randomly. The deep well plates were incubated for 6 hours and antibody production was induced with 0.5 % galactose. Cultures were incubated for additional 18 hours. The pH values were recorded every 10 minutes and the resulting pH profiles are shown in Fig. 3. Compared to the strain grown in the standard synthetic media, addition of increasing concentrations of buffering substance maintained the pH more stably; still the pH values of cultures buffered with 50 mM phosphate buffer dropped to values close to 5.
The final cell densities after 24 hours of growth indicated that the phosphate buffer has negative effects on growth, final OD600 values dropped by approximately 30 % from 8.61 in the standard synthetic media to 6.22 in the media buffered with 50 mM phosphate buffer (Fig. 3B). In contrast, final IgG titers in the cultures changed dramatically, a more than 5-fold increase in final antibody concentration was observed upon addition of phosphate buffer (Fig. 3C). The variations in final IgG titers among cultures with increased buffering capacity were minor. Overall, the addition of buffering substance increased the final IgG titers, though based on the measured pH profiles, the effect cannot be accounted solely on a more stable culture pH as at a concentration of 20 mM, the pH still dropped very rapidly.
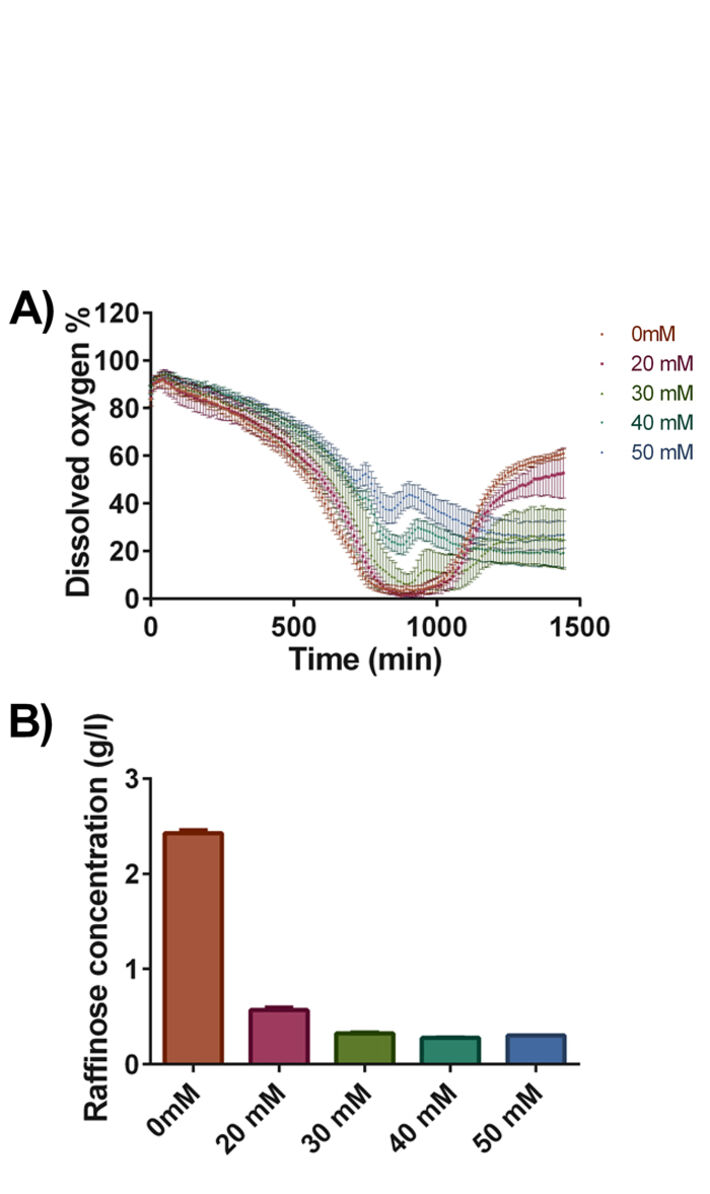
We wondered whether the observed positive effects were due to other metabolic changes. Therefore, using the identical experimental set-up we repeated the experiments using Deep Well OxoDishes® expecting that changes at the metabolic level could affect oxygen consumption and thus might be reflected in the dissolved oxygen concentration. The culture dissolved oxygen profiles are shown in Fig. 4A. Remarkably, the control culture and the culture buffered with 20 mM buffer had very distinct dissolved oxygen profile compared to the other cultures. After a drop of the dissolved oxygen to values close to 0 %, oxygen reached close to 50 % air saturation after 24 hours of cultivation. In contrast, the initial strong decrease in dissolved oxygen was less pronounced in the two cultures with 40 and 50 mM phosphate buffer due to lower OD. A second, intermediate oxygen decrease indicates a change of substrate. The following steady state between oxygen consumption and oxygen ingress stabilized slightly above 20 % air saturation. The cultures containing 30 mM phosphate buffer displayed an intermediate behavior where the rapid drop in dissolved oxygen level was followed by a change of substrate and a steady state resulting in DO values at about 30 % air saturation towards the end of the cultivation. The observed differences in dissolved oxygen concentration were accompanied by changes in the utilization of the carbon source raffinose (Fig. 4B). In the case of cells grown in the standard synthetic media raffinose consumption was lowest. Galactose, which serves as inducer, was completely consumed in all of the cultures. For the cultures with 30, 40 and 50 mM phosphate buffer the change in substrate consumption can clearly be seen in the DO kinetics as a second oxygen decrease after the first increase.
Conclusion
The application of online monitoring of systems such as Deep Well Oxo- and HydroDishes® is very useful to gain additional insight into culture conditions and cellular physiology. The data generated during the study confirmed the importance of buffering capacity of the media to ensure product stability. Unexpectedly, it also revealed that the buffering capacity of the media also can affect cellular physiology as evidenced by changes in oxygen consumption and use of substrate, thus opening up new research questions.
References
[1] Nasab, F. P., Aebi, M., Bernhard, G., Frey, A. D. 2013. A combined system to engineer glycosylation efficiency and glycan structure in Saccharomyces cerevisiae. Appl Environ Microbiol. 79: 997 - 1007
[2] Piirianen, M. A., Boer, H., de Ruijter, J. C., Frey, A. D. 2016. A dual approach for improving homogeneity of human-type N-glycan structure in Saccharomyces cerevisiae. Glycoconjugate Journal 33 (2): 189 - 199
[3] de Ruijter, J. C., Koskela, E. V., and Frey A. D. 2016. Enhancing antibody folding and secretion by tailoring the Saccharomyces cerevisiae endoplasmic reticulum. Microbial Cell Factories. 15:87
[4] de Ruijter, J. C., Jurgens, G., Frey, A. D. 2017. Screening for novel genes of Saccharomyces cerevisiae involved in recobinant antibody production. FEMS Yeast Res. 2017 Jan: 17 (1). fow104



