Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Online pH and DO Measurements in Microcarrier-Based hMSC Cultivations
Culture monitoring with sensor spots integrated in single-use spinner flasks
V. Jossen1, C. Schirmaier1, G. T. John2, D. Eibl1, and R. Eibl1
1Zurich University of Applied Sciences, School of Life Sciences and Facility Management, Institute of Biotechnology, Wädenswil, Switzerland
2PreSens Precision Sensing GmbH, Regensburg, Germany
Spinner flasks are often used for microcarrier-based cultivations of human mesenchymal stem cells (hMSCs). Normally, they are not equipped with pH and dissolved oxygen (DO) probes. This application note describes the cultivation of hMSCs in single-use spinner flasks equipped with optical pH (SP-HP5) and DO (SP-PSt3) sensors from PreSens for the first time. While reaching peak cell numbers between 4.1 x 107 cells and 5.9 x 107 cells in two cultivation runs, reliable DO and pH data were delivered.
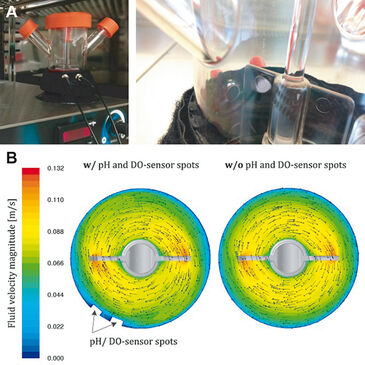
Spinner flasks are well established in cultivations of hMSCs at mL scale [1-3]. For example, screening of cell culture media, microcarrier types, and process parameters can be quickly carried out with spinner flasks [4]. But typically, they are not instrumented and allow no online measurement of key process parameters such as DO and pH, which have a strong influence on the cultivation results. The present study investigates the reliability of DO and pH values measured online with optical sensors from PreSens (Germany) during microcarrier-based expansions of adipose tissue-derived human mesenchymal stem cells (hADSCs). The optical sensors were attached to the inside walls of 125 mL spinner flasks (Corning, USA). The polymer optical fibers for sensor excitation and read-out were fixed in position opposite the sensor spots with an ARC (Adapter for Round Containers, PreSens) and connected to a pH-1 mini and OXY-4 mini for measurements (Fig. 1A). It is worth mentioning that conventional probes cannot be installed in the used spinner flasks due to the narrow space between the stirrer and the reactor wall. Therefore, compared to conventional probes the miniaturized size of the sensor spots is a major advantage. Moreover, PreSens single-use pH and DO sensors do not change the fluid flow behavior of the culture broth inside the spinner flask (Fig. 1B).
Materials & Methods
Cultivations were performed with cryo-preserved hADSCs, second passage obtained from a single informed and consenting donor. The cells were provided by Lonza Cologne GmbH and were cultured on commercially available polystyrene microcarriers in a serum-reduced medium (5 % FBS), which was specially designed by Lonza, USA. In parallel to the instrumented spinners, we worked with standard spinners (control spinners), which had no sensor spots implemented. According to the sampling intervals, offline samples were taken from the control spinners. Before inoculation with cells, 1 g of the polystyrene carriers (growth surface 360 cm2) were transferred into the spinner flask. Subsequently the serum-reduced, pre-warmed culture medium was added to reach a working volume of 115 mL. The microcarrier/medium suspension was stirred for three hours in order to soak the sensor spots and to allow the suspension to equilibrate. Afterwards, pH and DO were recalibrated and 1 x 106 cells were inoculated. No stirring was performed during the first four hours to promote cell attachment. Then the culture was agitated at 60 rpm in 37 °C, 80 % humidity, and 5 % CO2 for a culture period of 7 - 8 days. Sample triplicates were taken at different time points to determine cell densities with the NucleoCounter NC-100 (chemometec, Denmark). Measurements of glucose and lactate were performed with the Cedex Bio (Roche Diagnostics, Switzerland) [data not shown here]. Additionally, pH was measured offline (Mettler Toledo). The online pH value was recalibrated, if the offline measurement showed a deviation of 0.1.
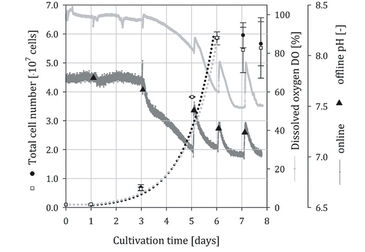
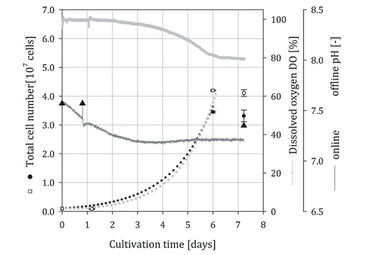
Stem Cell Culture in Spinner Flasks
In Fig. 2 and 3, the time-dependent profiles of the total cell numbers, pH (online / offline), and DO (online) measurements are given for two independent hADSC cultivations (squares = optical spinner, dots = control spinner). For all cultivations a one day lag-phase was observed. In cultivation run 1 a peak cell number of 5.95 x 107 cells was achieved at day 7 in the optical spinner. In the control spinner a peak cell number of 5.84 x 107 cells was measured on day 6. The simulated growth curves illustrate a comparable growth within the exponential growth phase for both spinner flasks. The cells grew with a mean growth rate (µ) of 0.83 d-1 (doubling time 20.1 h) comparable to results from previous investigations [4]. After achieving the peak cell numbers, further cell growth was presumably prevented by available microcarrier surface. In cultivation 1 (Fig. 2) the DO concentration decreased from 100 % to 52 %, whereby a specific oxygen consumption rate (qO2) of 4.7 x 10-17 mol s-1 per cell was calculated. Compared to literature data (2.50 x 10-17 [5] to 2.25 x 10-18 mol s-1 per cell [6]) the calculated oxygen consumption rate for the hMSCs was in typical range. The online measurement showed that the sampling on day 3, 5, 6, 7, and 8 had an impact on the cultivation system. The gas exchange was intensified during sampling, which resulted in an increased DO and pH signal (signal peaks). Nevertheless, the offline pH measurements showed similar values (mean deviation < 2 %) compared to online measurements. The minimal deviation indicates the reliability of the online measurements. Sampling in cultivation 2 was lessened (day 0, 1, 6, and 7) to reduce gas exchange. Accordingly, smoother signal curves without abrupt peaks for pH and DO were obtained. The peak cell numbers (3.45 x 107 cells in the optical spinner, 4.11 x 107 cells in the control spinner) in cultivation 2 were around 29 - 42 % lower than in cultivation 1. However, a comparable growth with a mean growth rate (µ) of 0.65 d-1 corresponding to a doubling time of 25.6 h was calculated for the optical spinner as well as for its control. The reduced cell growth was also obvious from the DO signal, where a decrease of 21 % (from 100 % to 79 %) was found. By using the estimated specific oxygen consumption rate (qO2) of cultivation 1, a mean cell number of around 4.14 x 107 cells could be predicted for cultivation 2.
Conclusion
These results demonstrate that online DO measurement can be used to assess and predict the cell growth in single-use spinner flasks. Moreover, the online pH measurements indicate that the reduced gas exchange causes a rapid decrease of the pH after inoculation, which may impair cell growth. The online pH and DO measurements with the optical sensors from PreSens allow accurate and reliable measurement of the two key process parameters.
Acknowledgement:
We want to thank the team of Dr. Christian van den Bos from Lonza Cologne (Germany) for providing cells, microcarriers, culture medium, and knowledge exchange.
References
[1] D. Schop, et al. (2008), J Tissue Eng Regen Med 4, 126 - 135
[2] S. Kaiser, et al. (2013), Chemie Ing Tech 85, 95 - 102
[3] C. J. Hewitt, et al. (2011), Biotechnol Lett 33 (11), 2325 - 35
[4] C. Schirmaier, et al. (2014), Eng Life Sci
[5] P. Godara (2008), J Chem Tech Biotechnol 420, 408 - 420
[6] Q. a Rafiq, et al. (2013), Biotechnol Lett 35 (8), 1233 - 45


