Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Process Monitoring in Suspension-Adapted CHO Cell Cultures
Non-invasive online detection of pH and oxygen
Harry Abts1 and Sarina Arain2
1Celonic GmbH, Karl-Heinz-Beckurts-Straße 13, Jülich, Germany
2PreSens GmbH, Josef-Engert-Str. 11, 93053 Regensburg, Germany
Online detection of oxygen and pH by using SDR at a small-scale can be used as a basis for latter cultivation in large-scale bioreactors to detect optimal growth conditions. Contamination-free online monitoring of culture parameters is not only important for scaling up production in process development, but it also could be valuable for other applications such as tissue engineering, stem cell research, and toxicological tests.
Suspension-adapted Chinese hamster ovary cell (CHO-S) cultures are widely used in biotechnological production of recombinant proteins. In fact, such special cell lines have become the standard for this type of biopharmaceutical production. The reasons for that include their fast reproduction, high protein expression rate compared with other eukaryotic cells and, above all, the glycosylation patterns generated by the cells. Efficient production of correctly processed proteins requires optimized cultivation parameters. The SensorDish® Reader (SDR) offers a non-invasive online detection method that for the first time enables quantitative characterization of both pH and oxygen in 24-well multidishes. From CHO-S cell cultures sampled in various start concentrations, a correlation can be demonstrated between cell number, oxygen consumption, and pH value during cell cultivation.
Monitoring CHO-S Cell Respiration
The CHO cells which were used in these experiments were from Celonic GmbH, Germany, who also performed this study. The cell line (CHO-S) was adapted to suspension culture. These cells display a high metabolic rate and grow serum-free to yield cell concentrations of up to 2x106 cells/ml. The cultivation in a defined medium was performed over 12 days under standard conditions in the incubator (5 % CO2, 95 % humidity and 37°C). Four different cell concentrations (50, 2x103, 2.5x104; and 3.2x105 cells/mL) were tested in parallel to investigate the sensitivity of the system with respect to the limit of detection regarding the cell numbers per sample. Medium changes were performed in intervals of 24 h. The cells were cultivated directly in the 24-well HydroDish® (for pH monitoring) and OxoDish® (for oxygen detection). The optical sensors are located at the bottom of each well and are read out non-invasively with the SDR. Two of the 24-channel readers were connected in parallel for simultaneous read-out of oxygen and pH, respectively. The OxoDish® and HydroDish® are each placed onto the SensorDish® Readers, which are kept in the incubator for the whole time of the cultivation. The SensorDishes® are only moved from the SDR for the daily media change. This ensures optimal conditions for the cells in the incubator for the whole time of their cultivation. Measurements were taken automatically in intervals of 10 min. One well with culture media, but without cells, served as reference sample. Every cell concentration was used with one well of the OxoDish® and HydroDish®, respectively, applying a sample volume of 1.2 ml / well.
Non-Invasive pH Monitoring
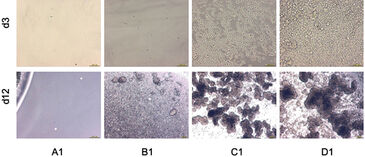
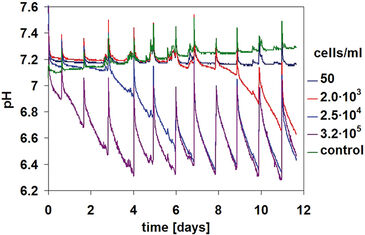
Figure 1 shows pH kinetics monitored during CHO-S cell cultivation. Depending on the initial cell concentration, pH values decreased at different speeds. The sample with the highest starting concentration (3.2×105 cells/mL) showed a significant pH decrease on the first day already, and that continued on the following three days with an upward tendency. The pH value decreased continuously from its initial value of 7.2 to a final pH of 6.3. Because of the daily media changes, a lower pH value was not obtained. Kinetics for samples with lower initial cell concentrations show similar behavior: pH values decreased after a delay of three days (for 2.5×104 cells/mL) and six days (for 2×103 cells/mL), respectively. And for the lowest starting concentration of 50 cells/mL, no significant pH change of the medium was observed. The start of the pH decrease therefore correlates directly with the beginning concentration of CHO-S cells, which is also shown in a microscopy photo (Figure 2).
Oxygen Consumption
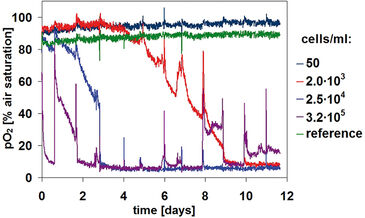
Oxygen consumption depends directly on both cell concentration and the metabolic state of a culture (Figure 3). For the highest cell density of 3.2×105 cells/mL, the oxygen content dropped from a starting value of 90% air saturation to only about 9% within several hours. This drastic effect was observed after each change of medium. After about five days, the oxygen content dropped to 6%, whereas from day 10 onward a slight increase was detected. The deviation in oxygen values after medium change after one day is assumed to be due to accidental removal of cells during the medium change procedure. Samples with lower cell concentrations show similar kinetics, with a delay of about 12 hours or four days, respectively. The oxygen uptake rate increases at each medium change. Because of the high metabolic rate of CHO-S cells, low oxygen levels are reached again rather quickly following a change of medium. Microscopic data regarding cell density and structure (Figure 2) give further information on the specific kinetics:
The two higher concentrations form cell aggregates over longer cultivation times (Fig. 2, wells C1 and D1 at Day 12). And that influences the oxygen kinetics in two ways. First, cell complexes can be removed during media change. Second, it is very likely that aggregate structures increasingly inhibit cellular metabolism, which is probably the reason for the oxygen increase to a level of 15% air saturation from day 10 onward. As in pH monitoring, metabolism of the lowest cell density culture is undetectable. For this set-up, the oxygen ingress into the sample from ambient air alone completely compensates for the oxygen consumption by the cells. Kinetics allow an estimation of a limit of detection of 8×103 cells/mL, assuming a doubling time of 35 hours for low cell densities. Oxygen levels at the start of cultivation lay within the OxoDish® resolution of ±5% air saturation. As with the pH sample, the control sample for oxygen measurement showed stable values for the duration of the cultivation.
Future Applications
This successful sensitivity test is the base for new studies with more sensitive cell types. These are e.g. human chondrocytes, ceratinocytes and hepatocytes, used for investigation in regenerative medicine. Another field is stem cell research. Here, exactly defined oxygen contents during the in vitro-development can be monitored and, if necessary, regulated. The detection of oxygen kinetics enables also new applications in pharmacology and toxicology, e.g. using HeLa cells or primary cell lines. The cytotoxicological effect of new active agents can be investigated fast and quantitatively.
Application note adapted from
BioProcess International (1/2008, 64-66 )