Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Revealing Oxygen Dependency of Bioluminescent TNT-Sensing E. coli K12 MG1655
High Resolution Oxygen Measurements with a Bare Fiber Optical Microsensor and OXY-1 ST
Chritian Ude1, Gernot T. John2, Marc Prante1, Rachel Rosen3, Shimshon Belkin3, Thomas Scheper1
1Institut für Technische Chemie, Gottfried Wilhelm Leibniz Universität Hannover, Hannover, Germany
2PreSens Precision Sensing GmbH, Regensburg, Germany
3University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem, Israel
Buried explosive material containing TNT represents a severe issue for human and animal safety. Its additional high toxic potential and harmful effects to the environment manifest the need to detect and dispose TNT and its derivatives from nature. It is possible to use a genetically modified E. coli K12 MG1655 bacterial strain to detect 2,4-dinitrotoluene (DNT), which is a decomposition product of TNT leaching even through sealed containers [1]. This strain is able to produce luciferase and emit bioluminescence after exposition to DNT. For an effective application of this strain in biosensors its dependency on external parameters had to be evaluated first. By application of the OXY-1 ST and a bare fiber oxygen microsensor it was possible to identify oxygen as the most crucial parameter for the cells' luciferase bioluminescence reaction and critical concentrations were determined. Furthermore, oxygen limitation and hydrogel-thickness-thresholds of immobilized bacteria could be analysed in high spatial resolution to improve biosensor development.
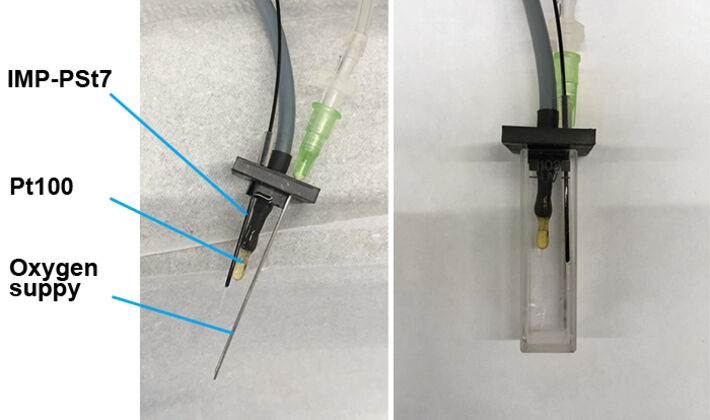
The OXY-1 ST used in combination with the implantable oxygen microsensor IMP-PSt7 allows dissolved oxygen measurement in extremely small environments with a detection limit of 0.03 % O2. The optical oxygen microsensors are based on a 230 µm silica fiber and have a sensor tip diameter of < 50 μm. We tested the performance of this device for two tasks:
1. Oxygen measurement in fused-silica cuvettes during bioluminescence measurements
For this task it was necessary to introduce several sensors and an aeration-nozzle into the cuvette without shading the detection window for bioluminescence measurements with a fluorescence spectrometer.
2. Measuring oxygen gradients in bacteria loaded agarose-hydrogels
For the second task dissolved oxygen had to be measured in spacings of 50 µm. Therefore, we needed an oxygen sensor with high spatial resolution.
Oxygen Monitoring in Fused-Silica Cuvette
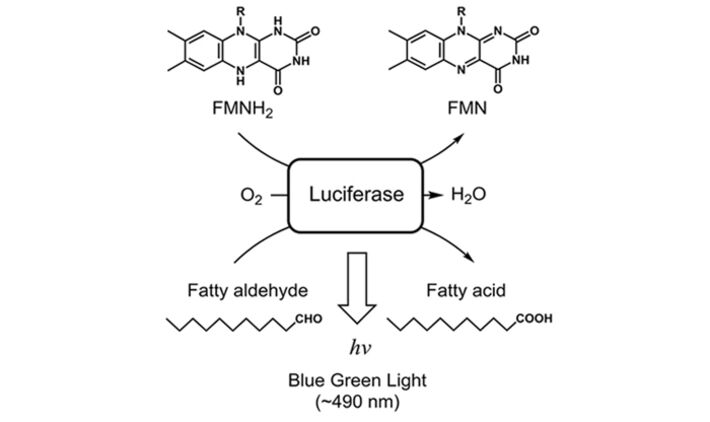
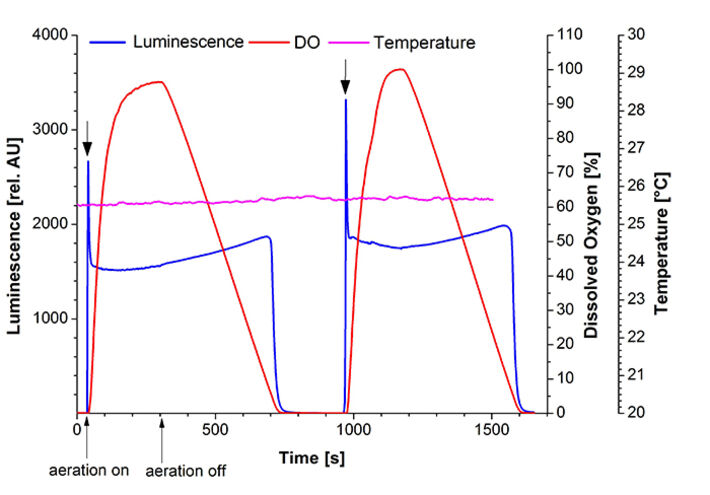
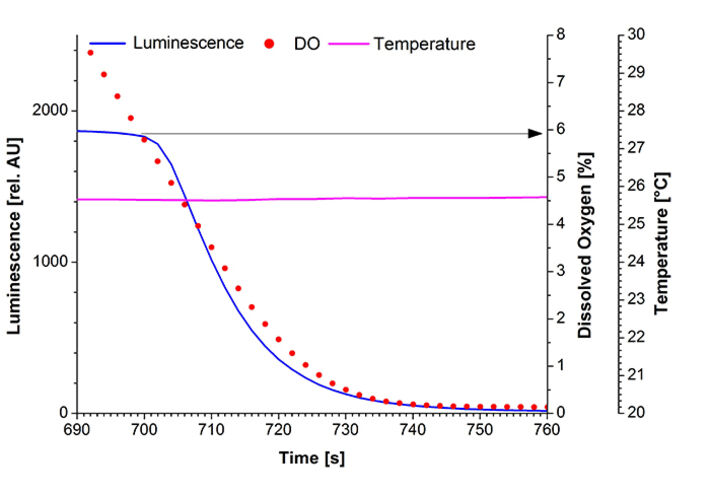
For the determination of luciferase parameter dependencies a standard fused-silica UV-cuvette was equipped with a Pt100 and the implantable oxygen microsensor IMP-PSt7, which was connected to the OXY-1 ST. A syringe-needle was introduced to control oxygen concentration in the liquid (Fig. 1). The cuvette was fixed in a fluorescence spectrometer (Hitachi F-7000) to measure total bioluminescence at λ = 480 nm. E. coli K12 MG1655 was cultivated in 500 mL baffled shake flasks in 100 mL LB-medium, at 180 rpm and 37 °C. The luciferase production was induced using 100 mg/L 2,4-DNT during exponential growth phase. Cells were harvested 3 h after induction, standardized to an optical density OD600 = 5 and transferred into a fused-silica cuvette. During measurement temperature was kept constant at 25.6 °C. At the beginning of the experiment no oxygen was available to the cells as can be seen in dissolved oxygen measurements (DO) and no bioluminescence was emitted at these conditions (Fig. 3). After 34 s oxygen supply was initiated at a flowrate of 5 mL/min. Within seconds a peak bioluminescence of 2665 AU was reached. In the following minutes luminescence stayed constant at 1500 AU while DO reached a maximum value of 96 % a. s. At this point the oxygen supply was turned off to further investigate the critical concentration for the luciferase mechanism. At 700 s of measurement bioluminescence started to decrease quickly (Fig. 4). At this point a critical DO-level of 6 % a. s. could be determined which is necessary to sustain bioluminescence emitted by the bacteria. The experiment was reproduced (t > 900 s) showing the same behavior. This oxygen dependency could also be supported by the reaction scheme of luciferase as shown in Figure 2.
Measuring Oxygen Gradients in Bacteria Loaded Agarose-Gels
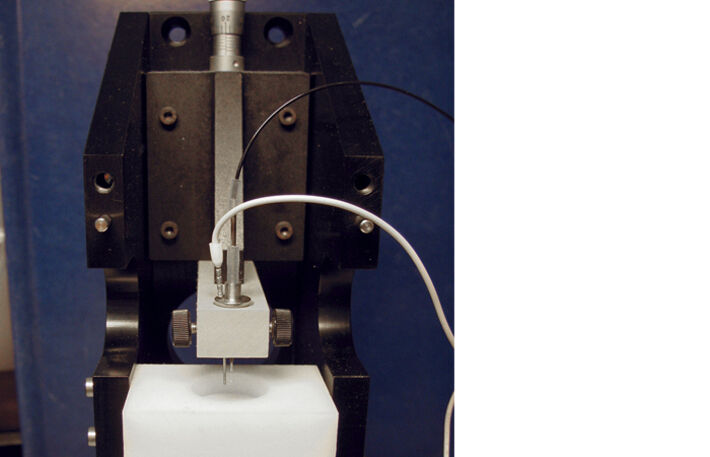
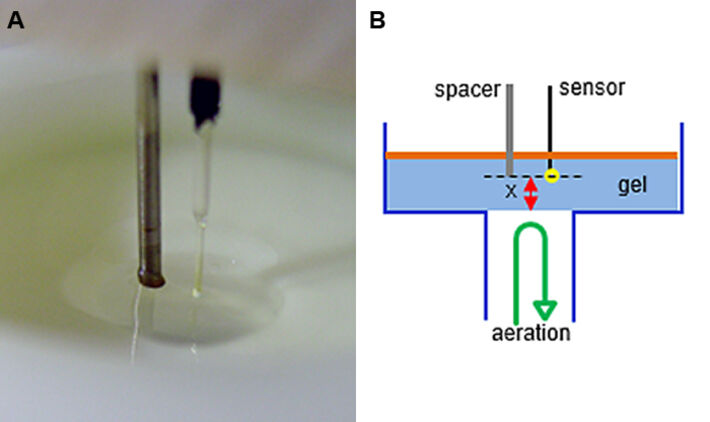
Since oxygen supply was determined to be crucial for luciferase function, this part investigates oxygen gradients in bacteria loaded agarose-gels. For this purpose, a micromanipulation unit was equipped with the oxygen microsensor IMP-PSt7 (Fig. 5). With this device the microsensor could be aligned precisely to a 'zero' position of a cylindrical casting form (Fig. 6). The bottom-hole in the form was used as air / oxygen supply channel and could be sealed by a metal stick for casting.
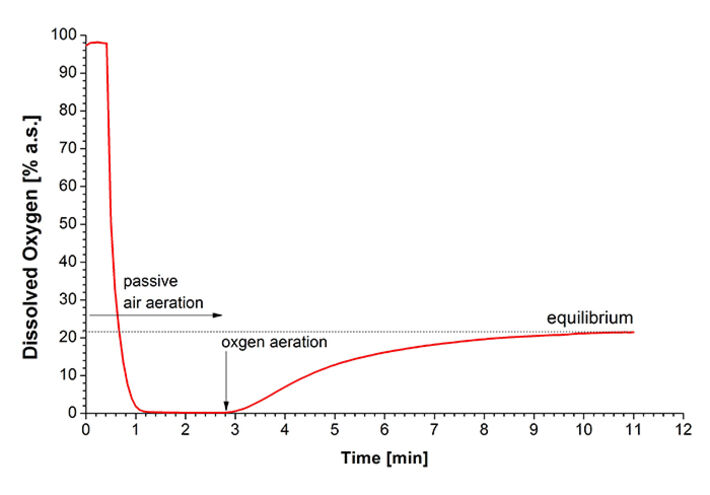
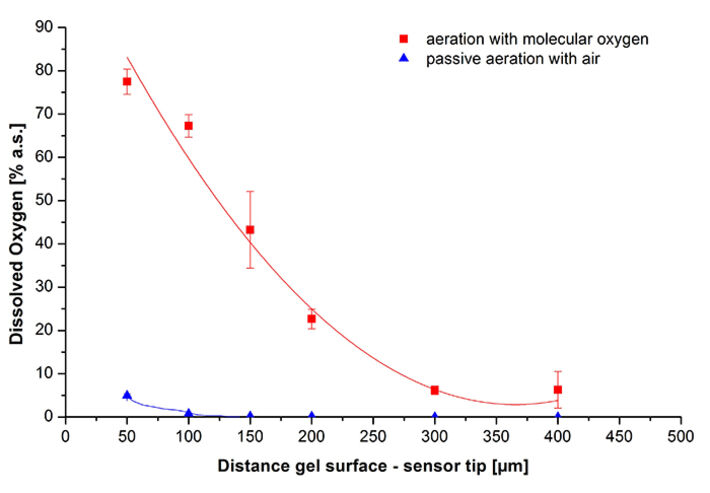
Cells for immobilization were harvested in the exponential growth phase, transferred into fresh medium and mixed with molten agarose-hydrogel to reach a final OD600 of 5 and an agarose content of 1 % (w/v). This mixture was cast into the form with the pre-adjusted microsensor IMP-PSt7 to obtain 2 mm thick hydrogels. After polymerization at 25 °C the gels was covered with paraffin oil. To enable gas-exchange the bottom-hole was aerated with either oxygen or air. As an example, Figure 7 shows DO measurement with gel-immobilized bacteria at a distance of 200 μm from gel surface to sensor tip. Once measurement was started a fast decrease of DO from 100 to 0 % took place due to respiration. At this depth no oxygen was detectable using passive air aeration, so the growth conditions passed to oxygen limitation. Aeration with molecular oxygen was started at 2.8 min at 10 mL/min to improve oxygen supply. An equilibrium of 22 % could be clearly reached after 11 min. These analyses were used to determine oxygen gradients at both aeration conditions (Fig. 8). It could be shown that passive air aeration is not sufficient to provide the beforehand determined critical DO level of 6 % a. s. at any tested invasion depth. Using oxygen aeration, it was possible to provide a sufficient oxygen concentration for both respiration and luciferase reaction at depths up to 400 μm at an OD600 of 5. That means gel disks can be casted up to 800 μm to provide sufficient oxygen for all immobilized cells.
Conclusion
Our experiments with the OXY-1 ST on a biophysical strain characterisation of the immobilized E. coli K12 demonstrated that this device can measure precisely in space-limited environments using the IMP-PSt7 microsensor. The high spatial resolution made it possible to precisely resolve oxygen gradients in hydrogels at steps of 50 μm. The fast response time (t90 < 3 sec.) enables to monitor fast changes in DO levels which can be necessary for monitoring enzyme reactions. Since the microsensor does not consume oxygen itself it is particularly suitable for monitoring changes at very low oxygen concentrations.
Application note adapted from
Prante M., Ude C., et al.: A Portable Biosensor for 2,4-Dinitrotoluene Vapors. Sensors (2018), 18, 4247
References:
[1] Yagur-Kroll, S., Lalush, C., Rosen, R. et al., Escherichia coli bioreporters for the detection of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol (2014), 98, 885 - 895
[2] Prante, M., A Bioluminescence Biosensor for 2,4-Dinitrotoluene, Masterarbeit, Institut für Technische Chemie, Leibniz Universität Hannover, 30.09.2017



