Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Testing Plant Seed Viability by Respiration Measurements
Oxygen Consumption Monitoring in 96 Well Plate with VisiSens TD and ViviPlate a Newly Developed Airtight Plate Clamp System
G. Liebsch & R. Meier
PreSens Precision Sensing GmbH, Regensburg, Germany
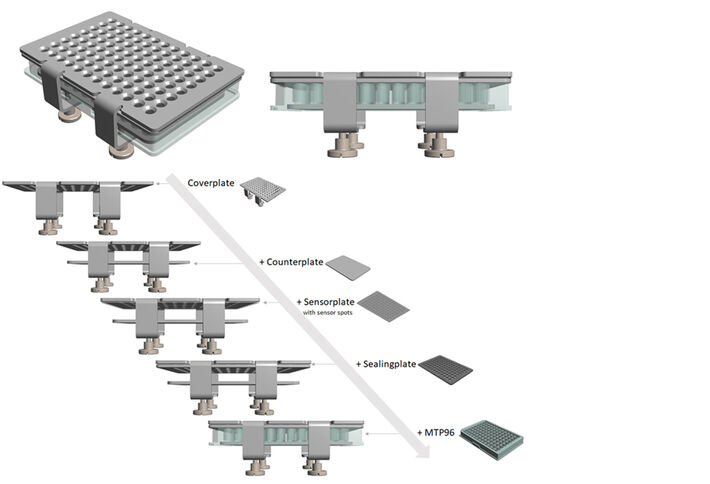
PreSens has developed the sensor and clamp tool ViviPlate that allows to create closed respirator chambers of different numbers and volumes in microtiterplate format. These chambers can then be read-out in parallel from above using our imaging system VisiSens. All chambers are closed in one step by covering with a reusable sensor equipped transparent sensorplate. A sealingplate and a coverplate with holes at the well/vial and sensor sites allow optical access to the fluorescent optical oxygen sensors from above. The sandwich is squeezed with knurled screws that press a counterplate against the coverplate ensuring airtight sealing of each well/vial.
ViviPlate allows Viability and Vitality testing of multiple samples via monitoring the oxygen consuming energy metabolism of mircroorganism, tissues or cells. For this, ViviPlate assesses the oxygen consumption rate (OCR). By adding our CO2 sensors ViviPlate will soon be available in a product version that allows to determine the respiratory quotient (O2 uptake / CO2 release).
In the following application, we show how to use this tool for plant seed viability testing in 96 samples of four different plant species. During germination plant seeds consume oxygen and seed respiration can be used as an indicator for viability. In our experiments we used the new sensor and clamp system ViviPlate, that allows to measure oxygen in the headspace of 96 well plates. Plant seeds of four different species were placed in the 96 well plate, one in each well. Optical oxygen sensors were positioned on top of each well and the clamp system ensured that the plate and each well were sealed off. The oxygen sensors were read out with the VisiSens TD system which allows to record all 96 oxygen sensors in each well in one image, and oxygen decrease caused by respiration during germination was recorded over time. The VisiSens ScientifiCal software then allowed to show oxygen consumption of all samples on one screen.
Materials & Methods
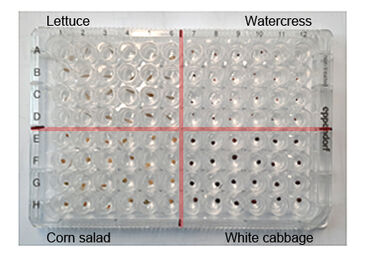
Each well of a standard 96 well plate was filled with 100 μL 1% Phytagel™ (Sigma-Aldrich®). In four sections of 24 wells each, one seed of four different plant species was placed in each well (lettuce, watercress, corn salad and white cabbage, Sperli GmbH; Fig. 3). All wells of the microtiterplate were closed by using a sealing with 96 holes and a transparent plate with attached and pre-positioned oxygen sensor spots (SF-RPSu4, PreSens). The 96 well plate with sealing and sensor cover was placed in a specially designed clamp (ViviPlate, PreSens) which can be fastened with knurled screws and ensures gas-tight sealing of the wells. The upper clamp plate has holes in the positions above the wells so the oxygen sensors can be read out from above. The fluorescent optical O2 sensors were read out with the VisiSens TD imaging system (PreSens) over a period of 3 days with an interval of 30 minutes. The oxygen recordings were visualized and analyzed with the VisiSens ScientifiCal software (PreSens).
Plant Seed Viability
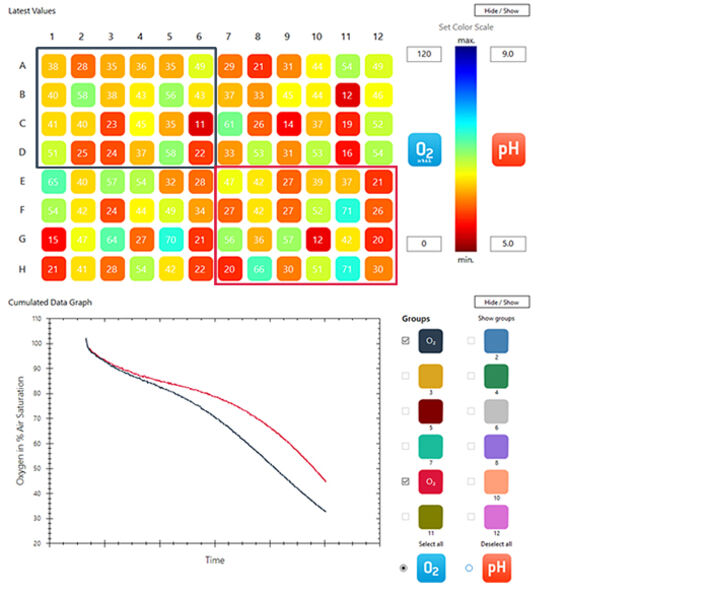
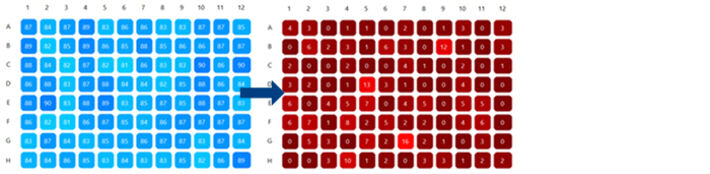
Figure 4 shows the oxygen measurements in the 96 well plate after 2 days. On top the oxygen concentration in each well is shown in a color representation. The color scale was set between 0 to 120 % air saturation as can be seen on the right. In some wells the oxygen concentration was still above 70 % air saturation, indicating that the seeds inside started germinating later compared to seeds in other wells, where O2 concentration had already decreased to values between 10 – 20 % air saturation. The software allows to group together certain wells and analyze the average O2 concentration in these wells. We selected the 24 wells with lettuce seeds (dark blue rectangle) and the 24 wells with white cabbage seeds (red rectangle) and put them in two groups. The time course of average oxygen concentration in these two groups is shown below in the Cumulated Data Graph in the respective group colors. For seeds of both species the average O2 concentration started to decrease shortly after the experiment was started. During the first few hours the oxygen decreased slower, but when germination set in in all wells the average O2 concentration dropped more rapidly. In figure 5 the starting and final oxygen concentrations in all wells is shown. All seeds germinated and consumed the oxygen in the headspace of the wells. In most wells the oxygen was already completely consumed (0 % air sat.) at the end of the experiment.
Conclusion
With the ViviPlate clamp and VisiSens TD oxygen imaging system we were able to assess plant seed viability in 96 samples in parallel. The system gives information about the oxygen content in each single well of a 96 well microtiter plate and not germinating seeds can easily be identified. The ViviPlate clamp securely seals the wells, so precise respiration measurements can be performed. The system is the ideal tool for oxygen monitoring in a multitude of samples in parallel.