Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Ischemia-related Cell Damage in Extracorporeal Preserved Tissue
Assessing new findings with a novel perfusion model using optical pO2, pCO2 and pH sensors
C. D. Taeger1, W. Müller-Seubert1, R. E. Horch1, K. Präbst2, F. Münch3, C. I. Geppert4, T. Birkholz5, A. Dragu1
Departments of Plastic and Hand Surgery1, Bioprocess Engineering2, Heart Surgery3, Pathology4, Anesthesiology5, Friedrich-Alexander-University Erlangen-Nuremberg, Germany
Tissue undergoing free transfer in transplant or reconstructive surgery always is at high risk of ischemia-related cell damage. This study aims at assessing different procedures using an extracorporeal perfusion and oxygenation system. Flow-through cells with integrated O2, pH and CO2 sensors were implemented in this system for online monitoring before and after the perfusate passed through a free muscle flap. Furthermore, the expression of HIF-1 alpha as marker for hypoxia and of the pro-apoptotic protein Caspase-3 in the skeletal muscle flaps was investigated, to assess improvements in tissue-conservation by the different treatments. Results show that an air saturated solution supplies sufficient oxygen to the free flap, and increased expression of HIF-1 alpha and Caspase-3 can be prevented by procedures conducted in this study.
Within the last decade numerous advances were made in the field of transplant medicine. In this context, not only organ preservation, but also extracorporeal tissue conservation has made enormous progress. In reconstructive surgery free skeletal muscle transfer represents a standardized microsurgical tool in case of e. g. reconstruction of traumatized extremities. However, complications and even complete failure due to partial or complete necrosis of the transplanted tissue may occur in 5 % of all cases. Tissue damage caused by prolonged ischemia after harvesting the flap is still one of the limiting factors. Ischemia describes a state of restricted or completely inhibited blood supply causing shortages in oxygen and nutrient supply. Low oxygen concentrations not only cause damage during time of ischemia, but also in the case of re-establishing circulation. Reintroducing oxygen as well as other components causes the release of free radicals that aggravates already developed damage. In the past different approaches were conducted to optimize tissue oxygenation during ischemia. At this time the standard procedure in reconstructive surgery for flap preservation is mostly treating them with a single flush of saline solution after harvesting, followed by cold storage until their reconnection to blood circulation. This study was set up to learn more about the influence of different parameters, which might affect the ischemia-related cell damage of the highly ischemia-susceptible skeletal muscle and to draw conclusions for potential implications in clinical routine.
Materials & Methods
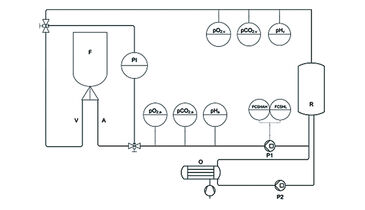
N = 24 muscle flaps were used in this study. After harvesting, the four flaps of group I were stored under room temperature without any further treatment (control group). Cannulas were inserted in the artery and vein of the five flaps in group II and connected to the perfusion and oxygenation system (Fig. 1, schematic model). The flaps of group II were perfused with a cardioplegic solution (histidine-tryptophan-ketoglutarate solution) for 60 min directly after harvesting. In group III, five muscles received an arterial needle and were treated with a singular flush of 10 mL of the cardioplegic solution directly after harvesting and then left untreated. In group IV, cannulas were inserted in five flaps both in the artery and vein to allow connection to our perfusion and oxygenation system. The flaps in group IV were perfused and oxygenated with a heparinised crystalloid fluid with an osmolarity of 291 mOsm/Liter (Jonosteril®). In group V, five flaps were perfused and oxygenated similar to group IV with heparinised cardioplegic solution. Constant perfusion was performed with a flow-rate regulating pump (Fig. 1, P1; Infusomat® Space P, Braun Melsungen, Germany) and a flow of 10 mL/min. Arterial and venous pressures were continuously monitored. The oxygenation with ambient air was performed through a neonatal oxygenator (Fig. 1, O; SAFE Micro®, Polystan, Denmark) in a secondary circuit. The primary circuit drew freshly oxygenated perfusate from the secondary circuit, which had an own pump (Fig. 1, P2). During active perfusion pO2, pCO2, and pH of the perfusate were continuously determined on arterial (Fig. 1, pO2,a, pCO2,a, pHa) and venous side (Fig. 1, pO2,v, pCO2,v, pHv) of the perfusion system. All parameters were determined using optical sensors integrated in flow-through cells for non-invasive data acquisition with fast response times, read out via polymer optical fiber and the respective fiber optic meter (OXY-4 mini, pCO2 mini, pH-1 mini, PreSens, Germany). Data acquisition was performed with a rate of 4/min. Using the flow-through cells with integrated sensors allows parameter determination without influence on flow rate and salt concentrations in the perfusates. To determine the influence of the different treatments on expression of Caspase-3 and HIF-1 alpha, a biopsy was taken 0 (baseline), 15, 30 and 60 min after explantation. Biopsies were stored in formalin at ambient temperature before embedding them in paraffin. One slide per staining method was taken from the prepared sample block.
Results
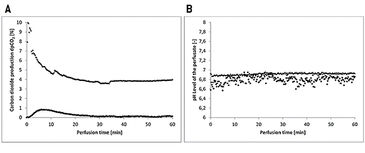
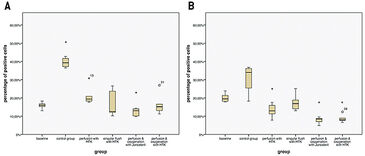
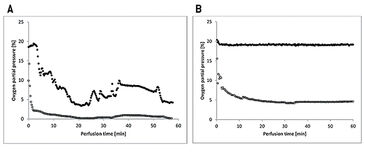
The flaps of the groups without oxygenation extracted the oxygen nearly completely during perfusion, as venous pO2 went down to approx. 0 % pO2 (Fig. 2A). In contrast, by supplying 21 % O2 (ambient air) via an oxygenator the arterial pO2 was stabilized and the pO2 at the venous branch remained at a level of 5 % (Fig. 2B). Oxygen uptake of the tissue also results in a different CO2 excretion into the perfusate (Fig. 3A). While the maximum difference from venous to arterial pCO2 in the perfusate of tissues without additional oxygen supply does not reach beyond 1 % the difference in pCO2 with oxygenation has an initial value of 10 % and still holds a medium level of 3.9 % after one hour of perfusion. Arterial and venous pH levels of the perfusate do not show any inconsistent values. Therefore, a medium value of the pH in the perfusate was used to compare perfusates in tissue with and without additional oxygen supply (Fig. 3B). Both curves show a slight in- crease in pH over the perfusion time of one hour. Histological analysis showed, that every treatment (groups II - V) has statistically significant lower expressions of Caspase-3 after 60 min compared to the control group (group I), whereby group IV (perfusion and oxygenation with a crystalloid solution) showed the best results (Fig. 4A). A statistically significant lower level of HIF-1 alpha compared to the control group (group I) after 60 min could be detected only in groups where the flaps were continuously perfused either with crystalloid or cardioplegic solution (groups II, IV, V). Comparison of the control group (group I) with the treated groups (groups II - V) confirms that all treatments (except singular flush with cardioplegic solution, group III) decreased the HIF-1 alpha expression (Fig. 4B).
Conclusion
Our data suggests that there are methods for preventing increased expressions of HIF-1 alpha and Caspase-3 when regarding conservation of free flaps. Non-invasive measurements with the flow-through cells integrated in our perfusion and oxygenation system and simultaneous monitoring of O2, pH, and CO2 gave valuable information on oxygen demand and CO2 excretion of the investigated muscle tissue, and perfusate pH. Taking together these findings, optimization in organ and tissue storage could be realized by simple means of a non recirculating infusion. An air saturated solution is capable to cover the oxygen demand of a free flap and as long as there is no recirculation an additional oxygenation is dispensable. Such methods could be implemented in clinical routine.
Application note adapted from
C. D. Taeger et al. (2014): Ischemia-related cell damage in extracorporeal preserved tissue - new findings with a novel perfusion model, J. Cell. Mol. Med. 18: 885 - 894.
DOI: 10.1111/jcmm.12238