Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Oxygen and pH Monitoring in Deep Well OxoDishes® and HydroDishes®
Assessing optimal growth conditions for shaken yeast culture

Thomas Grimm
BIOWORX, Berlin, Germany
In this study different yeast cultivations in deep well OxoDishes® and HydroDishes® were examined. Cultivations were carried out at varying volumes to assess optimal growth conditions in the 24-well plates, and to determine minimal volumes for sensor read-out with the SDR SensorDish® Reader at 200 rpm. Furthermore, batch and fed-batch media with different glucose content and feeding rates were evaluated. Results showed that in volumes from 1.25 mL/well to 3 mL/well oxygen and pH values could be read with the SDR, though with less volume a stronger noise-ratio in the measurements was observed. Stable measurement recordings could be performed in the deep well plates for 72 hours. Filling volumes of 1.25 mL and 1.5 mL showed optimal oxygen supply and best growth.
PreSens deep well OxoDishes® and HydroDishes® allow online oxygen and pH monitoring in shaken culture with the SDR SensorDish® Reader. In the following experiments the yeast Pichia guilliermondii was cultivated in these 24-well plates. Aim of this study was to evaluate the suitability of the deep well Oxo- and HydroDishes® for monitoring microbial culture, to examine growth profiles, and to determine the minimal filling volumes at which sensor read-out at a shaking speed of 200 rpm was possible.
Materials & Methods
The SDR SensorDish® Reader was used for online monitoring of oxygen and pH in yeast culture inside deep well Oxo- and HydroDishes® (OD24-DW, HD24-DW, PreSens) equipped with covers (Applikon) for homogenization of oxygen ingress and evaporation. The system was mounted in a thermoshaker (Gebhardt) using the System Duetz clamp system (Applikon, Fig. 1). Cultivations were carried out at 200 rpm and 50 mm amplitude. The fed batch medium EnPresso (Biosilta), and the batch medium YED were used with different culture volumes per well as follows: row 1 = 1.25 mL, row 2 = 1.5 mL, row 3 = 1.75 mL, row 4 = 2.0 mL, row 5 = 2.5 mL, and row 6 = 3 mL. The first experiment should show how P. guill. grows in EnPresso fed-batch medium at different culture volumes. In a second experiment yeast growth in batch medium YED with either 20 g/L or 60 g/L glucose was examined.
Microbial Culture Monitoring
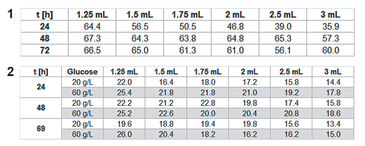
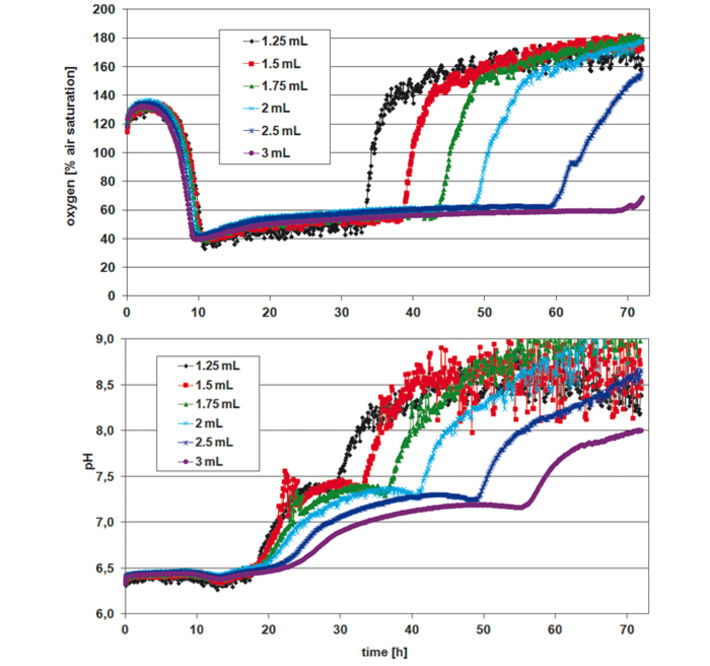
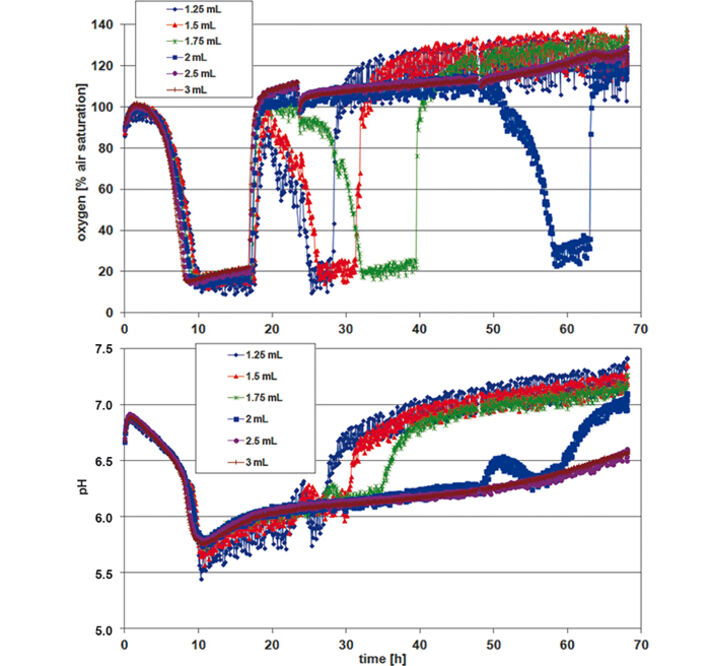
For assessing P. guill. growth in EnPresso medium the optical density (OD 600) was measured after 24, 48, and 72 h, calculating mean values for each row / culture volume (Tab.1). Optimal growth and oxygen supply showed at a filling volume of 1.25 mL. The maximum cell density was reached after 48 h. With increasing culture volume in the wells oxygen supply decreased and growth was slowed down. With smaller culture volumes, on the other hand, an increased noise-ratio in the oxygen and pH readings was detectable (Fig. 2). However, the kinetics can be compared quite well. The oxygen kinetics depict oxygen consumption for about 10 h; after that minimum oxygen values indicate high metabolic activity and growth. Due to varying oxygen ingress at different filling volumes the samples have different growth rates. Glucose is used up first for the samples with fastest growth (1.25 mL) after approx. 32 h, and last for the samples with slowest growth (3 mL) at about 70 h. This is indicated by an oxygen increase due to lower cell activity at constant oxygen ingress. The pH kinetics show a constant pH value of 6.5 for the first 18 h. After that, growth takes place at oxygen limitation, resulting in a pH increase to 7.5 due to consumption of complex medium components like peptone, yielding NH3. Again, after approx. 30 h kinetics change first for the samples with the highest growth (1.25 mL) and last for the ones with the slowest growth (3 mL). This is followed by a second pH increase. As the pH sensor has its upper measurement limit at pH 8.5, the values around and above this pH value are very noisy. When culturing P. guill. in YED batch medium at 2 different glucose contents optical density was determined after 24, 48, and 69 h (Tab. 2). Results showed better growth with medium containing 60 g/L glucose, reaching higher cell densities and growing over longer time periods. Again SDR measurements showed greater noise-ratio at lower culture volumes. Both oxygen kinetics with 20 and 60 g/L glucose show an oxygen decrease for 10 h (Fig. 3 + 4, top). However, samples with higher glucose content show longer and more stable growth at an oxygen minimum than samples with less glucose. Oxygen increases due to lack of glucose and therefore lower cell activity after 32 h in 60 g/L glucose. For low glucose medium oxygen increases after 18 h already. This is followed by a second decrease and increase, indicating consumption of a second substrate. pH decreases from about 6.8 to 5.5 for both glucose concentrations in the first 10 h. After this minimum, it goes up immediately to approx. 6.0 to 6.5. For high glucose, the pH stays at that value for 10 h and then starts to increase again slightly. Samples with low glucose show kinetics comparable to the oxygen graph: pH increases faster at 20 h for the highest cell concentration (1.5 mL), then drops and rises again, caused by NH3 production due to consumption of a second media compound. Comparing the fed-batch medium EnPresso with the batch medium YED, OD measurements using the same inoculum show better growth in EnPresso (data not shown). Oxygen kinetics look similar, provided that enough glucose is present in the batch medium. pH is constant in EnPresso for more than 10 h and decreases rapidly in the batch medium.

Conclusion
Stable measurement recording and reproducible microorganism cultivation can be performed in the deep well Oxo-and HydroDishes® on the SDR at 200 rpm shaking frequency. Lower sample volumes lead to higher OD due to higher oxygen ingress. The experiments showed that the optimal working volume for yeast cultivation in the deep well plates is 1.25 - 1.5 mL. Though measurements show a higher noise-ratio at low filling volumes valuable data can be gathered giving information about culture condition and media consumption.