Watch tutorials, webinars and informative videos about PreSens optical sensor systems.
Quality Assessment of Primary Human Keratinocyte Cultures
Online Measurement of Oxygen and pH with the SDR SensorDish® Reader
V. Lorenz, B. Roski, A. Hermann, and H. F. Abts
Celonic GmbH, Jülich, Germany
The SDR SensorDish® Reader allows non-invasive analysis of dissolved oxygen (DO) and pH in multiwell plates. In this sutdy the sensitivity of the monitoring device was tested in cell culture with keratinocytes (cell line and primary cells) showing comparatively low metabolic activity. Furthermore, it was investigated how cell proliferation would affect O2 and pH values inside the cultures. The correlation between cell proliferation analyzed with light microscopy, increase of oxygen consumption, and a decrease in pH value in the culture medium was clearly evident in the data recorded with the SDR. The oxygen and pH kinetics give valuable information about culture quality and efficiency, and prove the suitability of the SDR SensorDish® Reader for monitoring cell culture performance.
In vitro tests are standard screening processes in dermatological and cosmetic laboratories. New pharmacological substances can be tested on their efficiency, and quantitatively evaluated under defined conditions in culture vessels. In vitro cultivated epidermal keratinocytes have also become increasingly important in medical research, especially in tissue engineering - e. g. in transplantation of cultivated autologous skin cells for the treatment of burns which shows remarkable success rates. Efficient and reliable in vitro cell propagation is, therefore, extremely important and modern monitoring and control systems are applied to ensure reproducibility and quality of cell cultures. The PreSens SensorDish® Reader is a non-invasive, optosensoric monitoring system for long-term measurement of DO and pH in multiwell plates with integrated sensors. Even minimal changes in any of the two important growth parameters can be detected. The outstanding performance of this system has already been demonstrated in preliminary studies. The test described here focusses on the sensitivity of the SDR system used with cells showing comparatively low metabolic activity, and the relation of cell proliferation and oxygen consumption rates or changes in pH values.
Materials & Methods
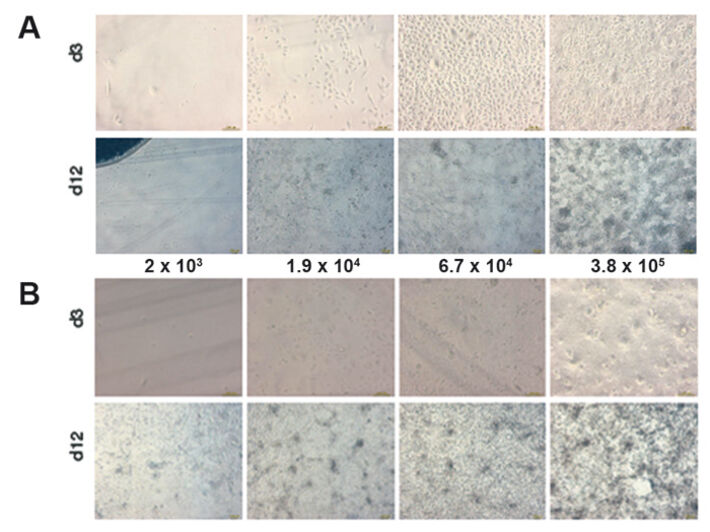
A spontaneously immortalized human keratinocyte celline (HaCaT-cells) and primary human keratinocytes were used for these experiments. Both cell types have a critical minimal cell density for inoculation. If this minimal value of cell density is not reached, cell growth is prolonged, or there is no growth at all. Both cell types were cultivated in parallel with four different starting densities of 2 x 103, 1.9 x 104, 6.7 x 104, and 3.8 x 105 cells/well. Reference measurements were performed in cell-free samples. 24-well Oxo- and HydroDishes® by PreSens were used as cultivation vessels and read out with the SDR SensorDish® Reader. The surface of the 24-well dishes was coated with collagen for the experiments, and for each cell density one well was analyzed online with the SDR. The culture period was 12 days. Cultivation was carried out at standard conditions in 5 % CO2 atmosphere with defined, serum-free keratinocyte-medium (Gibco Defined Keratinocyte-SFM). The medium was replaced every day with equilibrated, fresh medium.
Measurement in HaCaT-cell Culture
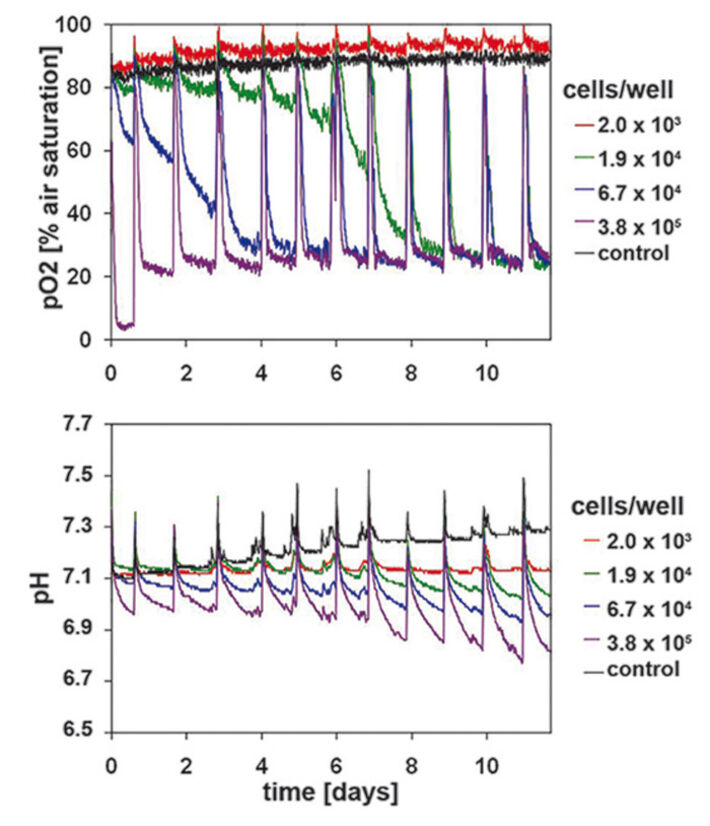
Measurements in epidermal HaCaT-cell culture were used as reference for primary keratinocytes. The sensitivity of the SDR system allowed detecting even smallest changes in the hardly metabolic active HaCaT-cells - even with starting cell densities of only 1.9 x 104 cells/well (Fig. 2). Lower starting cell densities showed no difference to values measured in the cell-free control wells. A continually increasing oxygen consumption rate could be measured for the cell culture inoculated at 6.7 x 104 cells/well. On day 6 this culture reached the same DO values as the culture seeded with highest cell density. Therefore, the ideal starting cell density for HaCaT culture is 6.7 x 104 cells/well. High proliferation and metabolic activity can be deduced from the rapidly decreasing DO level to minimum values within a few hours after media change. Similar results could be observed for the simultaneously recorded pH values (Fig. 2). The decrease in pH values correlated closely with the respective starting cell density. The pH values continually decreased over the whole culture period of 12 days. Cultures with highest starting cell density showed a pH value of 6.8 after 12 days of cultivation. Contrary to DO kinetics differences caused by starting cell density could still be clearly detected towards the end of the culture period. Cell proliferation was analyzed after 3 and 12 days of cultivation (Fig. 1A) and correlated with the SDR readings.
DO and pH Kinetics of Primary Keratinocytes
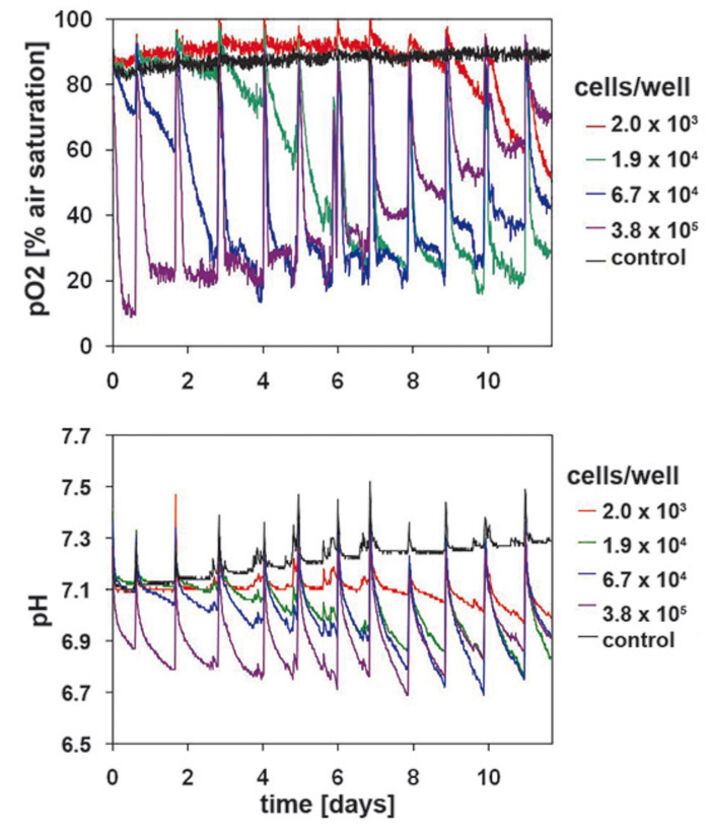
Oxygen consumption in primary keratinocytes (Fig. 3) is similar to that of HaCaT-cells at the beginning of the cultivation, but showed noticeable differences with continuing cultivation time. The sample with lower starting cell density of 2 x 103 cells/well showed significant metabolic activity from day 7 on, unlike the HaCaT-cells. Primary keratinocytes can tolerate low initial cell densities better than the HaCaT-cells. Cultures with high starting cell densities reached a minimum DO level of 22 % after only 3 days. After an initial minimum of 10 % an upward trend could be detected on day 1. Different from HaCaT-cell culture this increase continued after day 5. DO values stabilized at a higher level after each medium change. Towards the end of the cultivation period DO reached a value of 70 % air saturation. Other cell concentrations showed the same tendency with a 3 day postponement. This is a significant difference to the measured values in HaCaT-cell cultures, which allows the conclusion, that primary keratinocytes gradually reduce their metabolic activity. However, the noticeable decrease in oxygen consumption is not related to reduced viable cell numbers (Fig. 1 B). Primary keratinocytes show distinct contact inhibition compared to the HaCaT-cells. After forming a monolayer cells stop proliferating and the metabolism is reduced. Another reason can be the limiting culture conditions caused by high cell density, towards which primary keratinocytes react more sensitive than HaCaT-cells. The pH kinetics (Fig. 3) for different inoculation sizes showed the same tendency as DO kinetics. In all cultures pH values decreased after each media change, but this lessened from day 8 on. Even in the culture with lowest starting cell density a pH value decrease could be detected after day 7, which became larger till the end of the cultivation period. These effects are associated with the cell number and activity of the primary keratinocytes, and were most obvious towards the end of the cultivation period. Again the ideal starting cell density was 6.7 x 104 cells/well.
Conclusion
Precise insight in metabolic conditions within the culture vessels allows for new and promising perspectives in optimization of cell culture processes. The recorded kinetics give proof, that the SDR system is ideally suited for monitoring of sensitive keratinocyte cultures. Even at low initial cell densities DO and pH kinetics can be clearly determined. With these graphs detailed statements about culture development can be deducted. This way defined cultivation times can be set, according to process objectives, in which cultures show optimal physiological conditions, as for example for the further use in transplantation or for pharmaceutical screening tests. Future up-scaling processes may be based on the results gathered with the SDR in 24-well format.